Haemoglobin
Laura Armstrong & Joe Wolfensohn
Teachers


Contents
Recall Questions
This topic requires prior knowledge of protein structure. You can test your knowledge on this below.
Describe the primary, secondary, and tertiary structures of a protein.
-
Primary structure: Sequence of amino acids in a polypeptide chain, held by peptide bonds.
-
Secondary structure: Folding of the polypeptide into α-helices and β-pleated sheets, stabilised by hydrogen bonds.
-
Tertiary structure: Further folding into a specific three-dimensional shape, maintained by hydrogen bonds, ionic bonds and disulphide bonds between the R groups of the amino acids.
What is the quaternary structure of a protein?
It refers to a protein composed of two or more polypeptide chains joined together, sometimes with prosthetic (non-protein) groups.
What is the function of haemoglobin in red blood cells?
Haemoglobin transports oxygen from the lungs to tissues for aerobic respiration.
Topic Explainer Video
Check out this @JoeDoesBiology video that explains haemoglobin or read the full notes below. Once you've gone through the whole note, try out the practice questions!
Structure of Haemoglobin
-
Quaternary protein: Haemoglobin is composed of four polypeptide chains (two α-globin and two β-globin chains).
-
Prosthetic group: Each polypeptide chain contains a haem group, which includes an iron (Fe²⁺) ion that binds oxygen.
-
-
As there are 4 haem groups and 4 iron ions, each molecule of haemoglobin can carry 4 oxygen molecules when saturated.
-
-
Globular protein: Haemoglobin is water-soluble, allowing it to dissolve in blood plasma for efficient transport in the blood.
Human Haemoglobin

Role of Haemoglobin
- Haemoglobin has an affinity for oxygen.
- Haemoglobin associates with oxygen in the lungs.
- Haemoglobin disassociates with oxygen in the tissues.
The ability of haemoglobin to associate with oxygen and dissociate from oxygen depends on the concentration of oxygen in the surrounding tissues
Concentration of oxygen is measured as partial pressure pO2
Partial pressure – the pressure contributed by one gas in a mixture of gases.
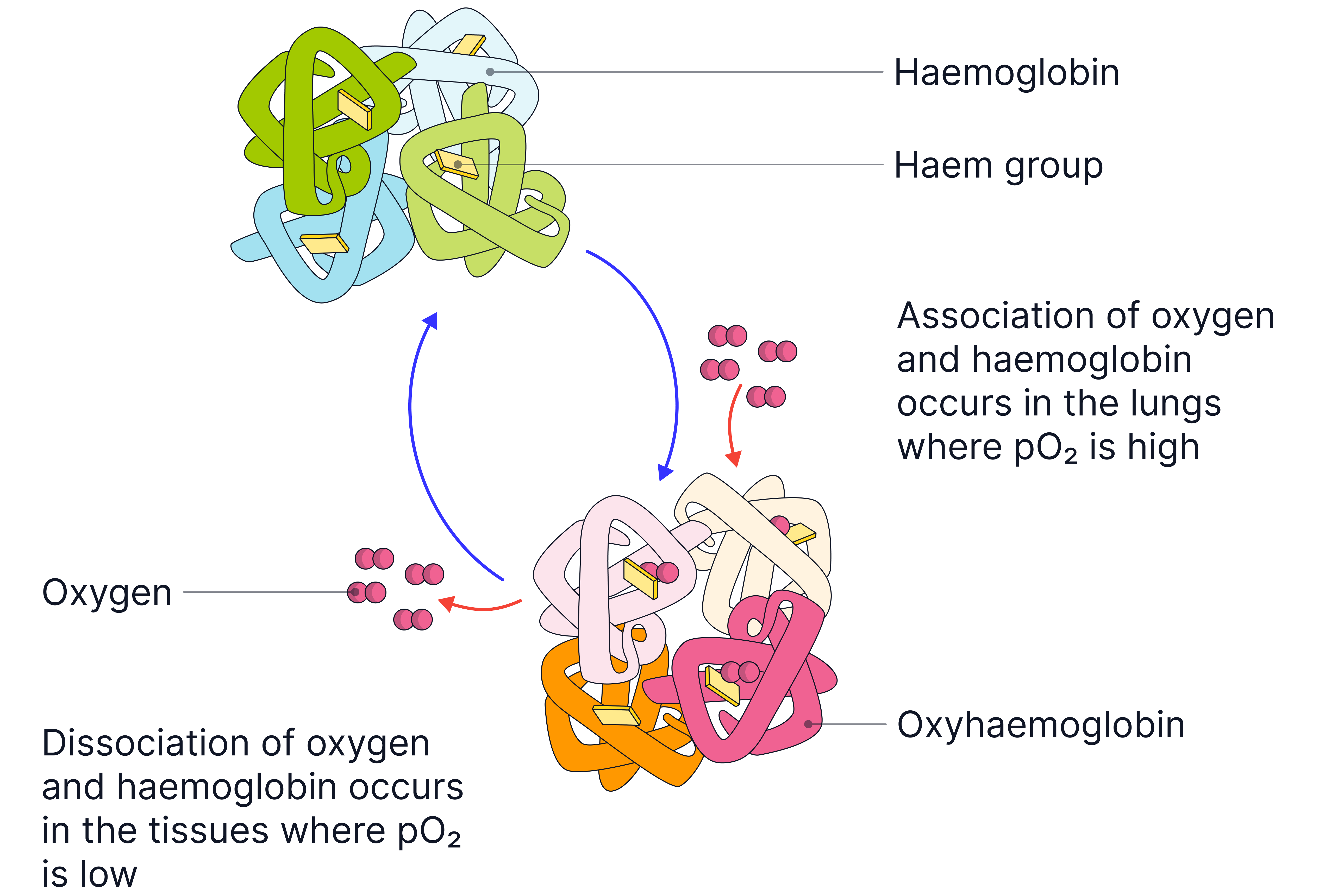
-
Oxygen transport: Haemoglobin loads or associates with oxygen in the lungs.
-
This is because there is a high partial pressure of oxygen in the lungs.
-
Haemoglobin unloads or dissociates with oxygen at the respiring tissues.
-
This is because there is a lower partial pressure of oxygen in the respiring tissues.
The Oxygen Dissociation Curve
Haemoglobins saturation with oxygen depends on the partial pressure of oxygen (pO2). This can be shown on an oxygen dissociation curve.

pO2 affects haemoglobin saturation as follows:
-
Higher pO2 - Haemoglobin has a high affinity for oxygen and loads with it (in the lungs).
-
Lower pO2 - Haemoglobin has a low affinity for oxygen and unloads it (at the respiring tissues).
-
At high pO2 (lungs), haemoglobin is almost fully saturated.
-
At low pO2 (tissues), oxygen is readily unloaded.
What is Co-operative Binding?
Co-operative binding refers to a property where the binding of one molecule to a protein influences the binding of subsequent molecules. In haemoglobin, this applies to the way oxygen (O₂) binds to its four haem groups.
How Does It Work in Haemoglobin?
Remember, haemoglobin is a quaternary protein made up of four polypeptide chains, each containing a haem group with an iron (Fe²⁺) ion that can bind one oxygen molecule.
-
First Oxygen Binding
-
Initially, haemoglobin has a low affinity for oxygen.
-
The first oxygen molecule binds with difficulty because the haem groups are less accessible.
-
Second and Third Oxygen Binding
-
Once the first oxygen binds, haemoglobin undergoes a conformational shift (the tertiary and quaternary structure changes).
-
This makes the remaining haem groups more accessible and increases haemoglobin’s affinity for oxygen.
-
The second and third oxygen molecules bind more easily.
-
Fourth Oxygen Binding
-
-
It becomes harder for the fourth oxygen molecule to bind, simply due to probability. The majority of binding sites are occupied so it is less likely that a single oxygen molecule will find the fourth binding site.
-
Maximum saturation is 100%. This would mean all haemoglobin proteins are associated with 4 oxygen molecules.
-
Key Terms:
-
Haemoglobin (Hb): A quaternary protein in red blood cells that transports oxygen.
-
Haem group: Iron-containing component that binds oxygen.
-
Oxygen dissociation curve: Graph showing haemoglobin saturation at different oxygen partial pressures.
-
Cooperative binding: A process where oxygen binding to one haem group increases affinity for additional oxygen molecules, making it easier for them to bind.
Exam Tips
Use correct terminology, such as ‘partial pressure of oxygen’ instead of ‘oxygen levels.’
When using oxygen dissociation curves, be prepared to talk about the percentage saturation of haemoglobin at different partial pressures of oxygen.
Binding of one molecule of oxygen to haemoglobin makes it easier for a second oxygen molecule to bind. Explain why. (2 marks)
-
Binding of the first oxygen molecule changes the tertiary / quaternary structure of haemoglobin.
(You could also say there is a conformational shift caused)
-
This uncovers a second / another binding site on haemoglobin making it easier for the second oxygen molecule to bind
(You could also refer to a second haem group or iron ion being uncovered instead of saying binding site).
Practice Question 1
Try to answer the practice question from the TikTok on your own, then watch the video to see how well you did!
Practice Question 2
If you want to try out another one, check this video out and see how you do!