Discovery of the Nucleus
Brook Edgar
Teacher

Contents
Explainer Video
Models Of The Atom
The model of the atom has changed over time. The earliest model of the atom was proposed by Dalton who thought that atoms were solid spheres that could not be divided any further.
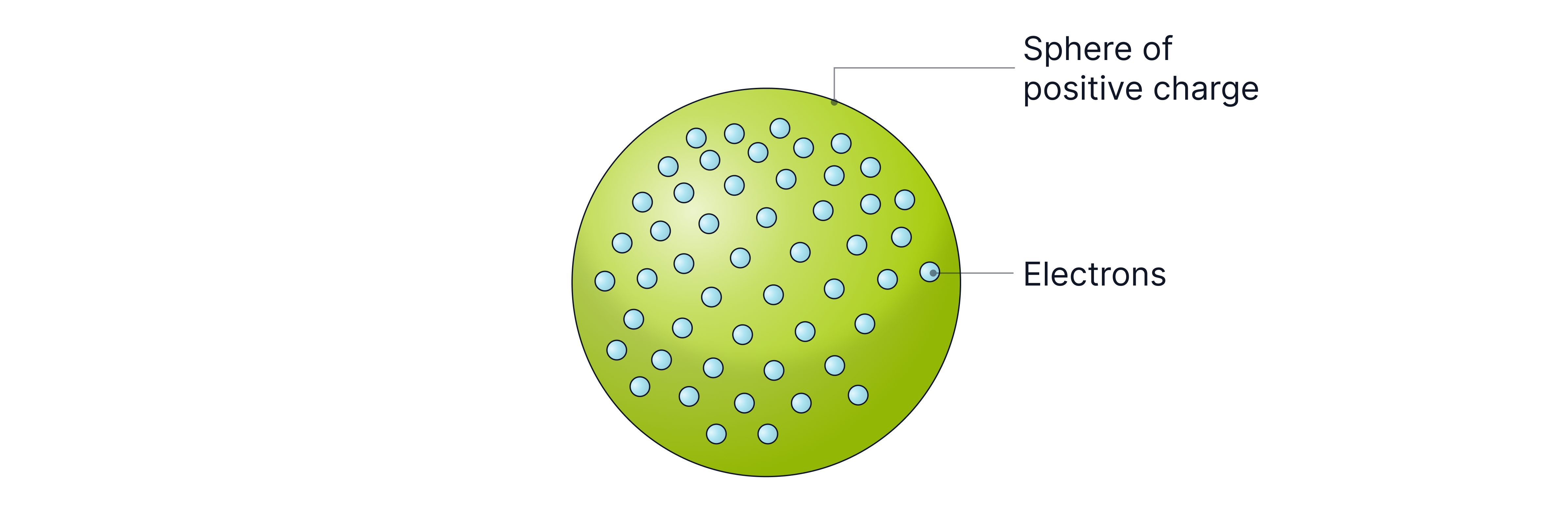
J.J. Thomson developed a new model called the plum pudding model. He suggested that the atom was a sphere of positive charge with negative charges distributed throughout. He named these negative charges electrons.
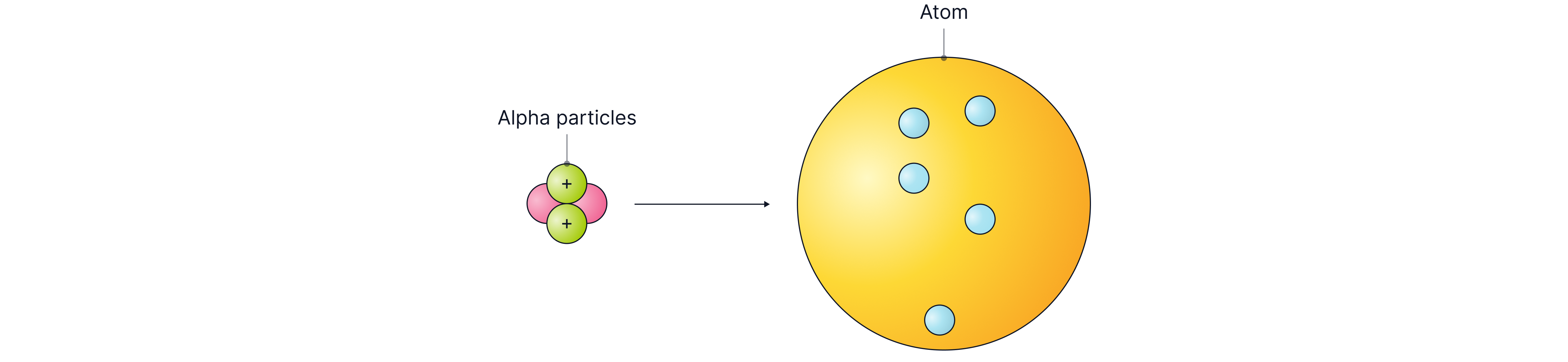
To test these models, scientists fired alpha particles (positively charged particles) at gold atoms and discovered that most alpha particles passed straight through the atom. This showed that most of the atom is in fact empty space. Some alpha particles bounced straight back or were deflected at angles greater than 90 degrees, leading to the conclusion of a positively charged nucleus.
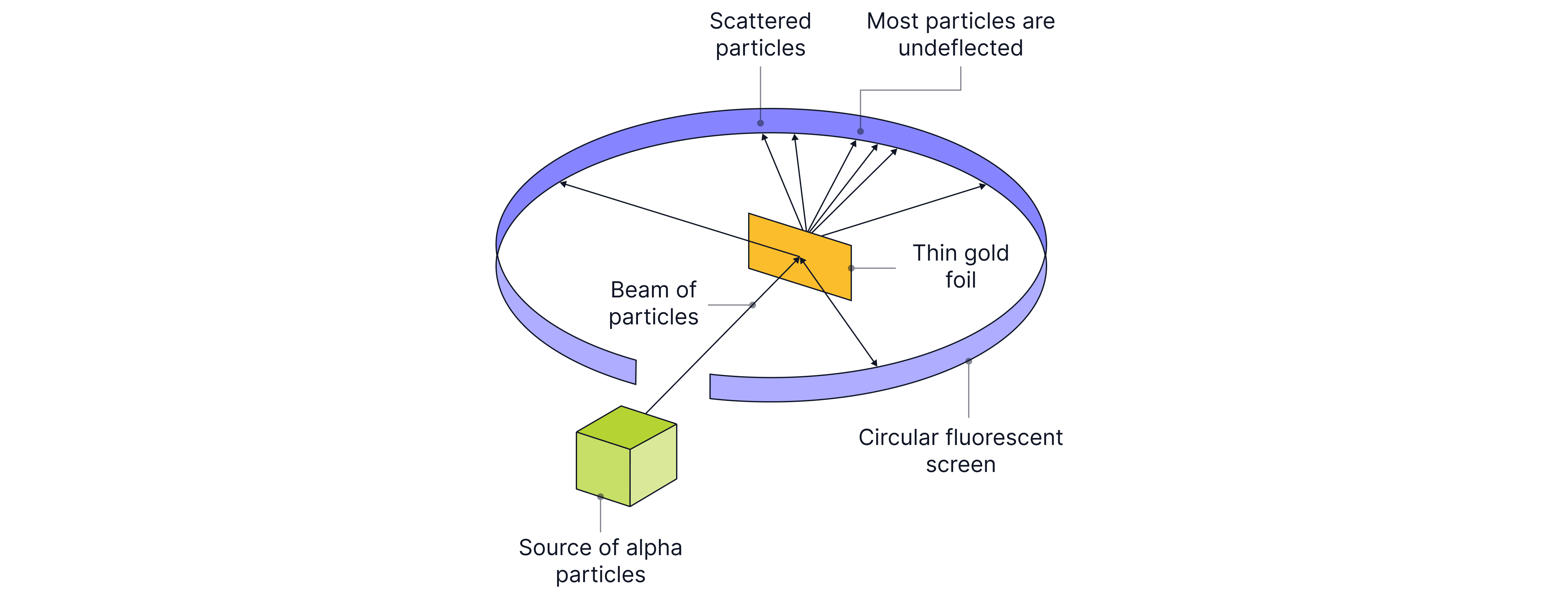
The alpha particles fired at the atoms all had the same kinetic energies, as otherwise slow-moving alpha particles would be scattered more than fast ones. The experiment was conducted in a vacuum, so there were no collisions with air particles. They used an alpha source with a long half-life, as otherwise, later readings could be lower than earlier ones.
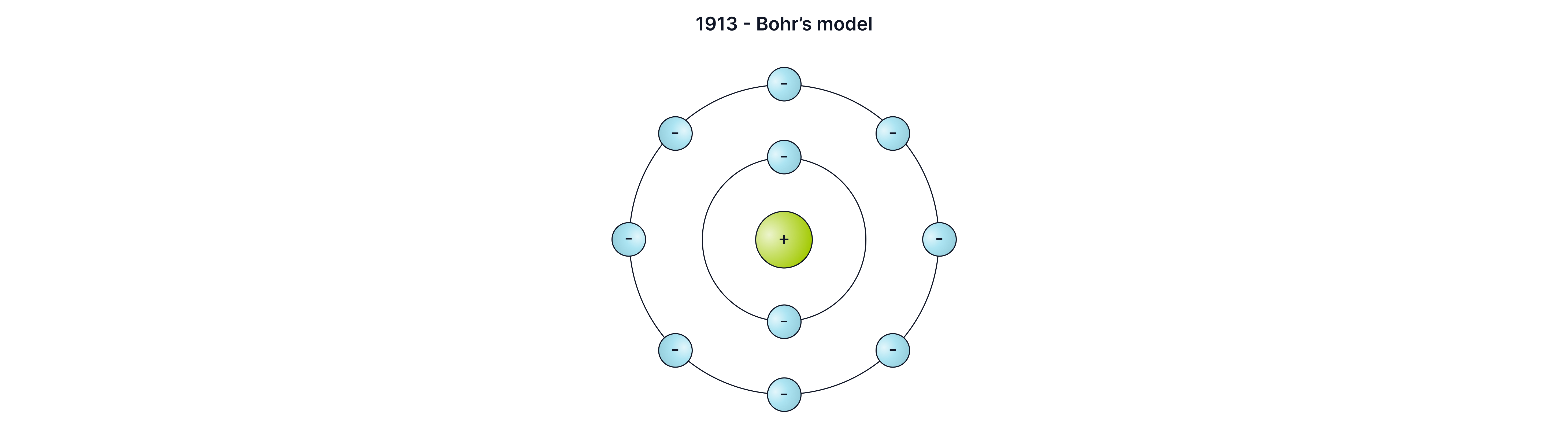
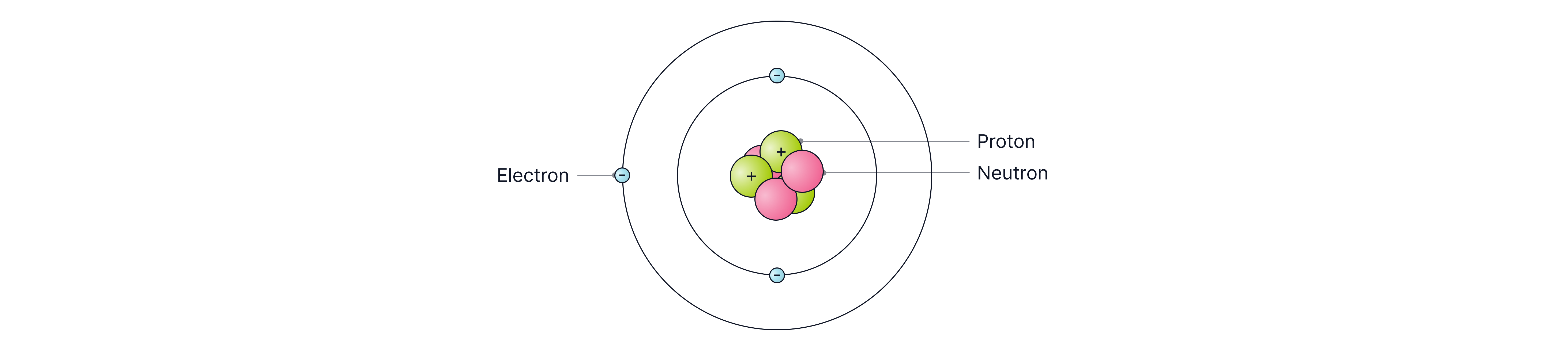
Later, Bohr found that electrons orbited the nucleus at specific distances, in certain energy levels (shells).
Then, scientists found that the nucleus could be divided even further and that the positive charge of the nucleus was due to small particles called protons. Chadwick also found that the nucleus contained neutrons, which had no charge.
Types of Radiation
The table below lists the types of radiation that you need to know and memorise.
Radiation is emitted from a large unstable nucleus randomly.
Type of Radiation | Also Known As... | Absorbing Materials | Range in Air |
Alpha
| Helium nucleus containing two protons and two neutrons. | Thin sheet of paper. | |
Beta Minus Beta Positive | Fast moving electron emitted from the nucleus or Fast moving positron emitted from the nucleus. | Aluminium sheet (about thick. | |
Gamma
| Electromagnetic wave/photon. | Thick lead sheet, concrete (more than thick) | Unlimited, spreads out in the air without being absorbed, follows the inverse square law. |
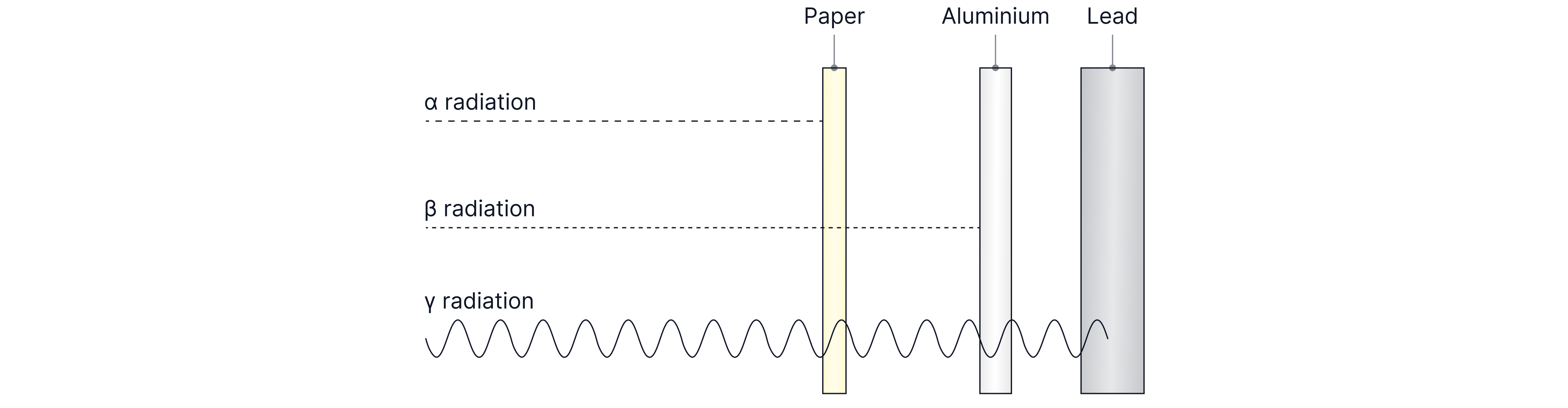
Alpha is the most ionising radiation as it produces the most ion pairs per centimetre of air. It is the most ionising as it is the largest and has the greatest charge. Ionisation occurs when an alpha particle collides with an atom, knocking off an electron, causing the atom to become an ion.
Worked Example
Alpha and beta particles lose about of kinetic energy in each collision they make with an air molecule. An alpha particle makes about collisions per cm with air molecules, while a beta particle makes about collisions. What is the range of an alpha particle and a beta particle if both start with an energy of ?
Answer:
An alpha particle makes collisions in total. Every cm in air it travels it undergoes collisions, so the total distance travelled by the alpha particles is:
An beta particle makes collisions in total. Every cm in air it travels it undergoes collisions, so the total distance travelled by the beta particles is:
Types of Decay
The nucleus is held together by the strong nuclear force as the electrostatic force of repulsion between the positively charged protons tries to tear the nucleus apart. If these forces become out of balance, the nucleus is unstable and will decay.
Beta-minus decay occurs if the nucleus contains too many neutrons - one of the neutrons in the nucleus changes into a proton (in Topic 5, we learned how to balance these equations due to conservation laws).
If the nucleus has too many protons, it decays by beta positive decay, where a proton changes into a neutron or by electron capture, where the nucleus captures an orbiting electron.
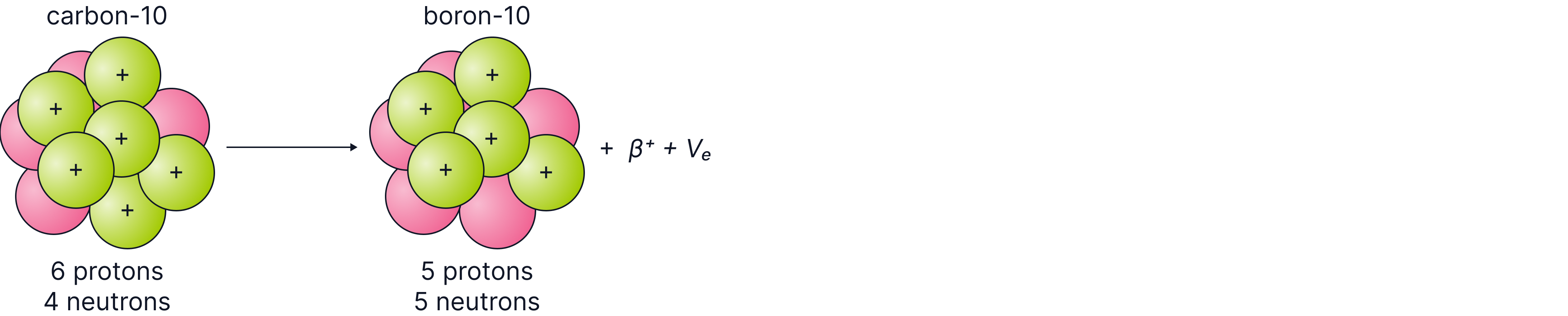

If the nucleus has too many nucleons, it will decay by alpha emission.
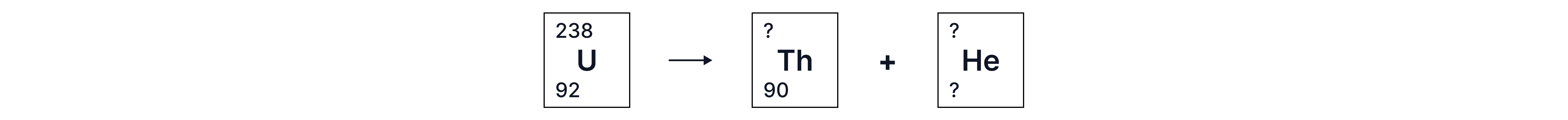
If the nucleus has too much energy the nucleus will emit a gamma ray (this usually occurs after a different type of decay, as the nucleus is excited and has excess energy).
Practice Questions
In JJ Thomson determined the specific charge of an electron.
Describe one method to do this.
-> Check out Brook's video explanation for more help.
Answer:
To find specific charge, we need an experiment that separates particles due to their mass and charge.
.
We know that moving particles in magnetic fields experience a centripetal force when the applied magnetic field is perpendicular to the velocity of the particle. By accelerating particles to the same speed, we can see that the point the particles hit the detector is due to their specific charge only.
The chlorine- isotope can be written as .
Calculate the specific charge of a Chlorine - atom.
Calculate the specific charge of a Chlorine - nucleus.
-> Check out Brook's video explanation for more help.
Answer: