Fluorescence
Brook Edgar
Teacher

Explainer Video
Energy Levels and Excitation
There are different energy levels within an atom where an electron can exist. The lowest energy level is called the ground state, represented as the energy level. Electrons can become excited and move to a higher energy levels. This is occur if the electron absorbs energy in the form of a photon with energy exactly equal to an energy level gap within the atom. Electrons can also be excited by colliding with a free electron and taking some of its kinetic energy. The excited electron is unstable, so it will eventually fall back down to a lower energy level in a process known as de-excitation. When de-excitation occurs a photon is emitted by the atom. The photon emitted will have an energy equal to the energy level gap through which the electron transitioned.
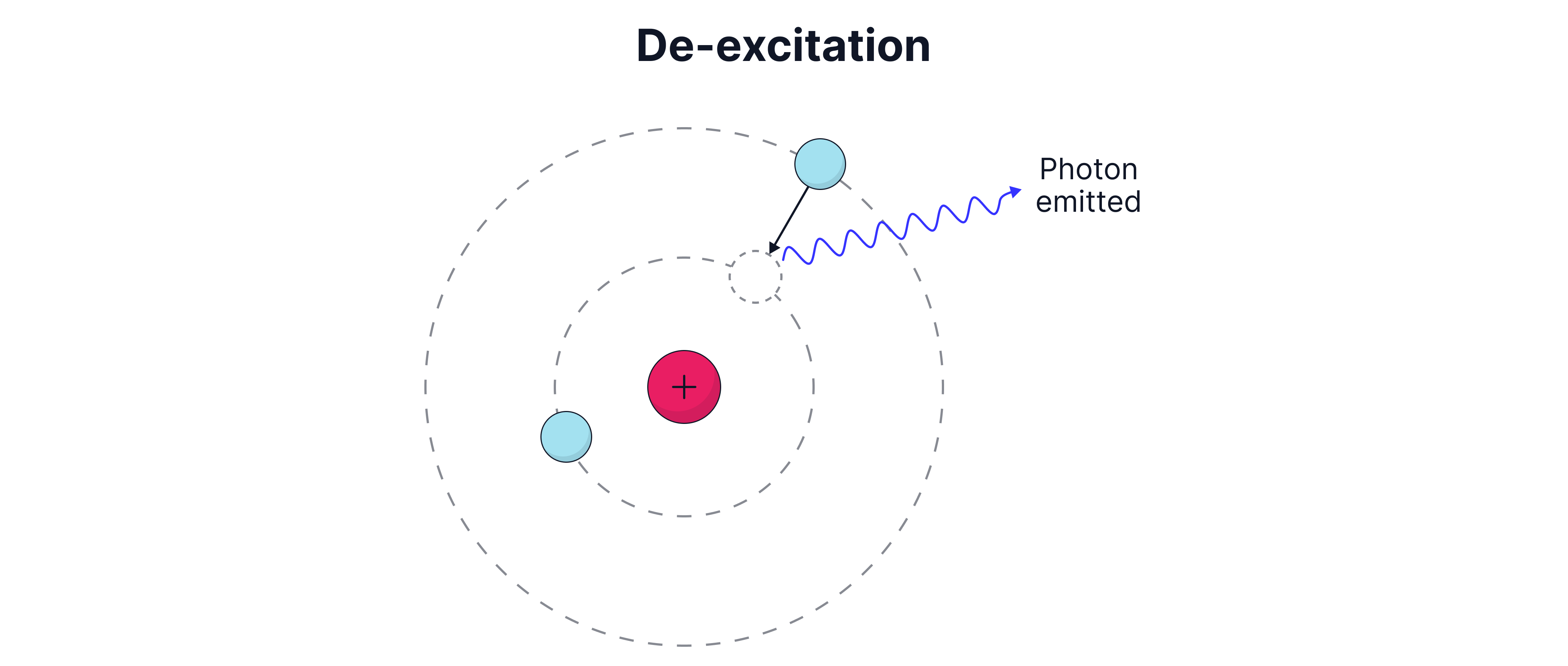
Worked Example
An electron is excited from the ground state to the second excited state. The electron then de-excites, falling from the n=3 energy level to the n=2 energy level and then back to the ground state.
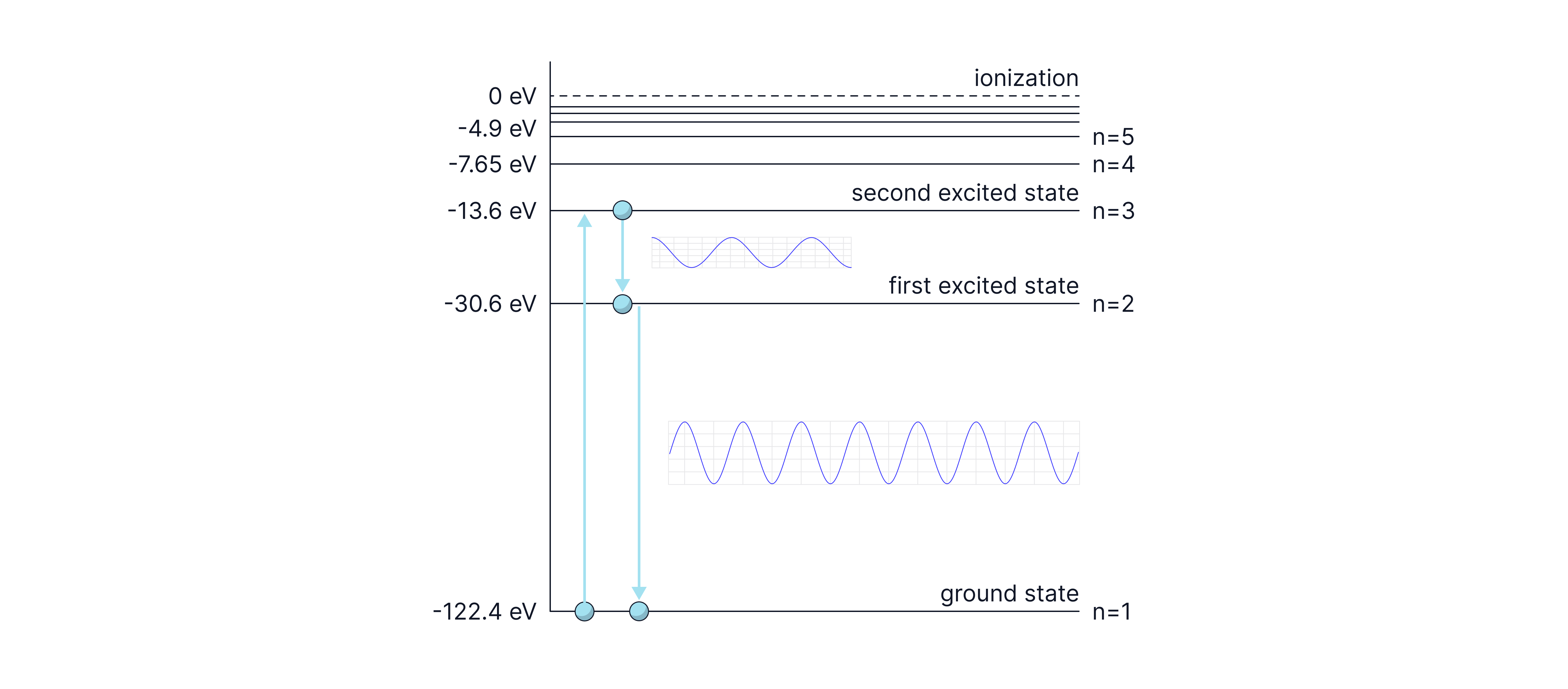
Calculate the wavelength of the two photons of light emitted during this de-excitation.
Calculate the ionisation energy in joules.
Answer:
First, we need to calculate the energy difference between the two energy levels within the atom.
We can now calculate the wavelength of light emitted as it will have an energy equal to this energy level gap.
*to convert from eV into joules, you multiply by the charge of an electron.
To free the electron from the ground state, eV is needed. A free electron is not bound to the atom, so it has zero energy. The bound states have negative energies, as energy has to be put into the system to remove the electron from the attraction of the positively charged nucleus.
Different atoms have different energy level gaps within the atom. We can use absorption and emission spectra to determine the atoms present in a sample of gas, as specific energies of light will only be absorbed/emitted depending on the size of the energy level gaps within the atom.
A blackbody, like a star, absorbs and emits all wavelengths of radiation (a continuous spectrum of light).
When this radiation passes through a cool gas, it causes the electrons within the atoms in the gas to become excited from their ground state. The electrons absorb specific parts of the continuous spectra, equal in energy to their energy level gaps. We can then use this absorption spectrum to determine the atoms present in the gas by comparing it to known reference spectra from the lab.
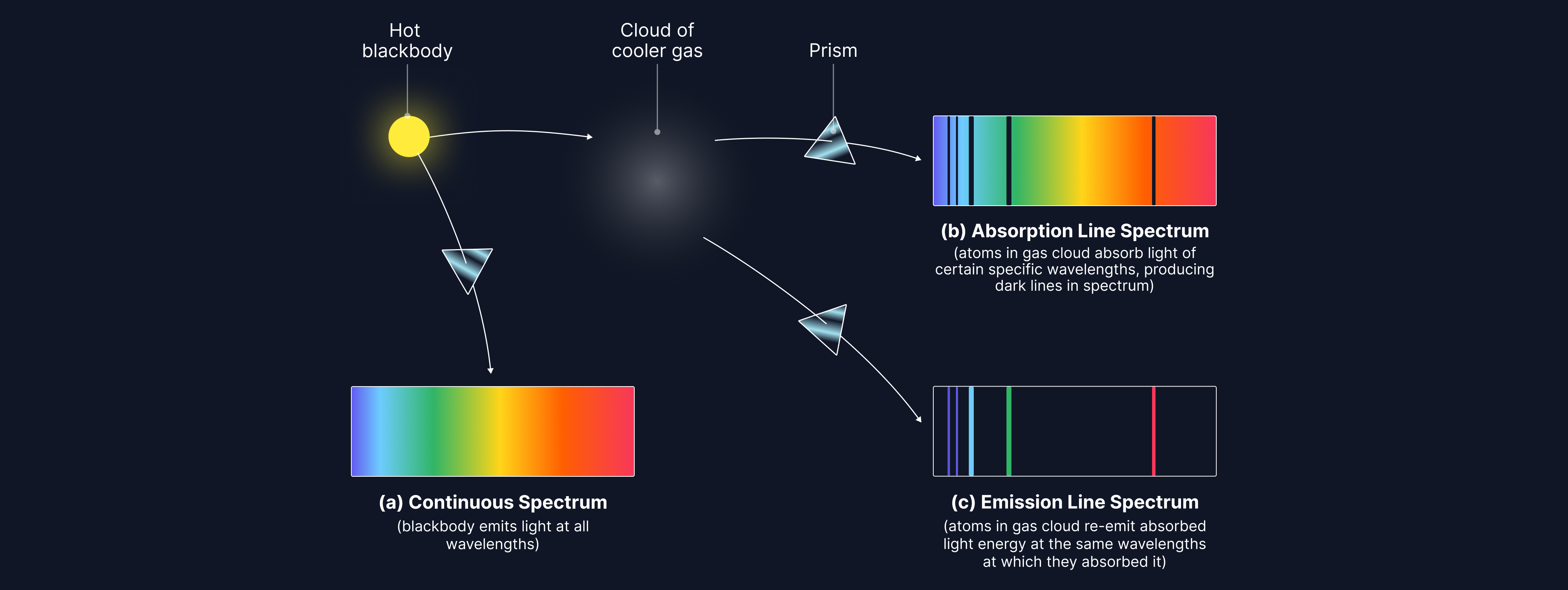
Later, the excited electrons will de-excite, emitting wavelengths of light equal in energy to the energy level gaps within the atom. This is known as an emission spectrum. Longer wavelengths of light are emitted when electron transitions occur between two energy levels that are close in value, and shorter wavelengths of light are emitted for large energy level transitions within the atom.
Reminder: In the visible spectrum, colours can be remembered using the acronym ROYGBIV -> Red, Orange, Yellow, Green, Blue, Indigo, Violet, from lowest to highest energies.
Fluorescent Tube
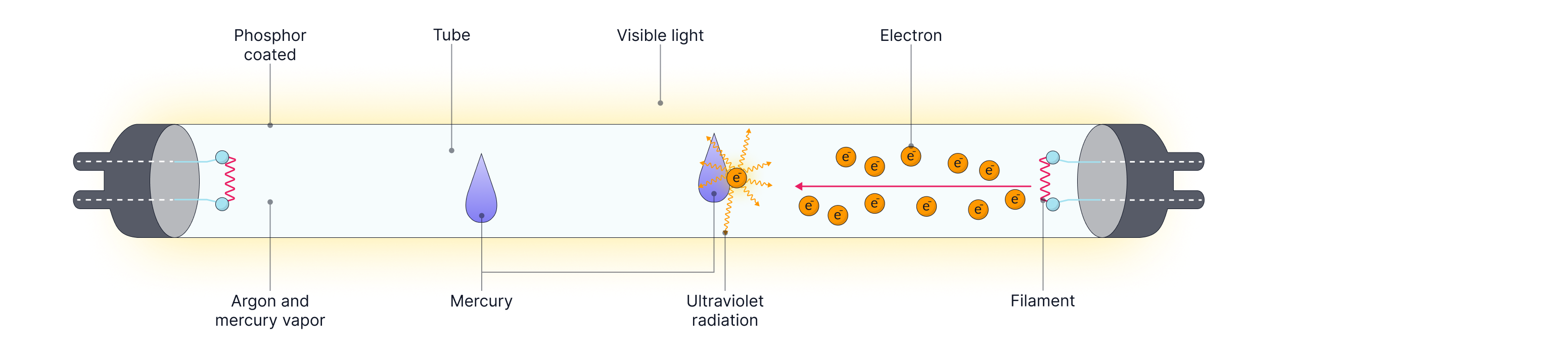
A Fluorescent tube works by applying a high voltage across the tube, which accelerates electrons from the hot filament (cathode, negatively charged plate) towards the anode (positively charged plate) through a low-pressure mercury gas.
*PANIC - can be used as an acronym to remember that Positive is the Anode, Negative Is the Cathode.
Electrons collide with the mercury atoms in the tube, transferring energy to the electrons within the mercury atoms, exciting them to higher energy levels. The electron within the mercury atom then de-excites, falling back down to a lower energy level and emitting a UV photon with energy equal to energy level gap the electron moved down.
The inner surface of the tube is coated with a phosphor coating. The electrons within the phosphor atoms absorb the UV photons and are excited to higher energy levels. These electrons then de-excite, falling back down to lower energy levels in stages, emitting photons of light with energy corresponding to smaller energy level gaps. ( Energy level gaps in phosphor atoms is smaller than in mercury). These photons all have energies in the visible part of the spectrum, allowing them to be seen.
Practice Questions
A gas initially has electrons within atoms in the n=4 energy level that de-excite to the n=1 level.

How many frequencies of light are observed?
A) 3
B) 4
C) 5
D) 6
-> Check out Brook's video explanation for more help.
Answer:
D) 6
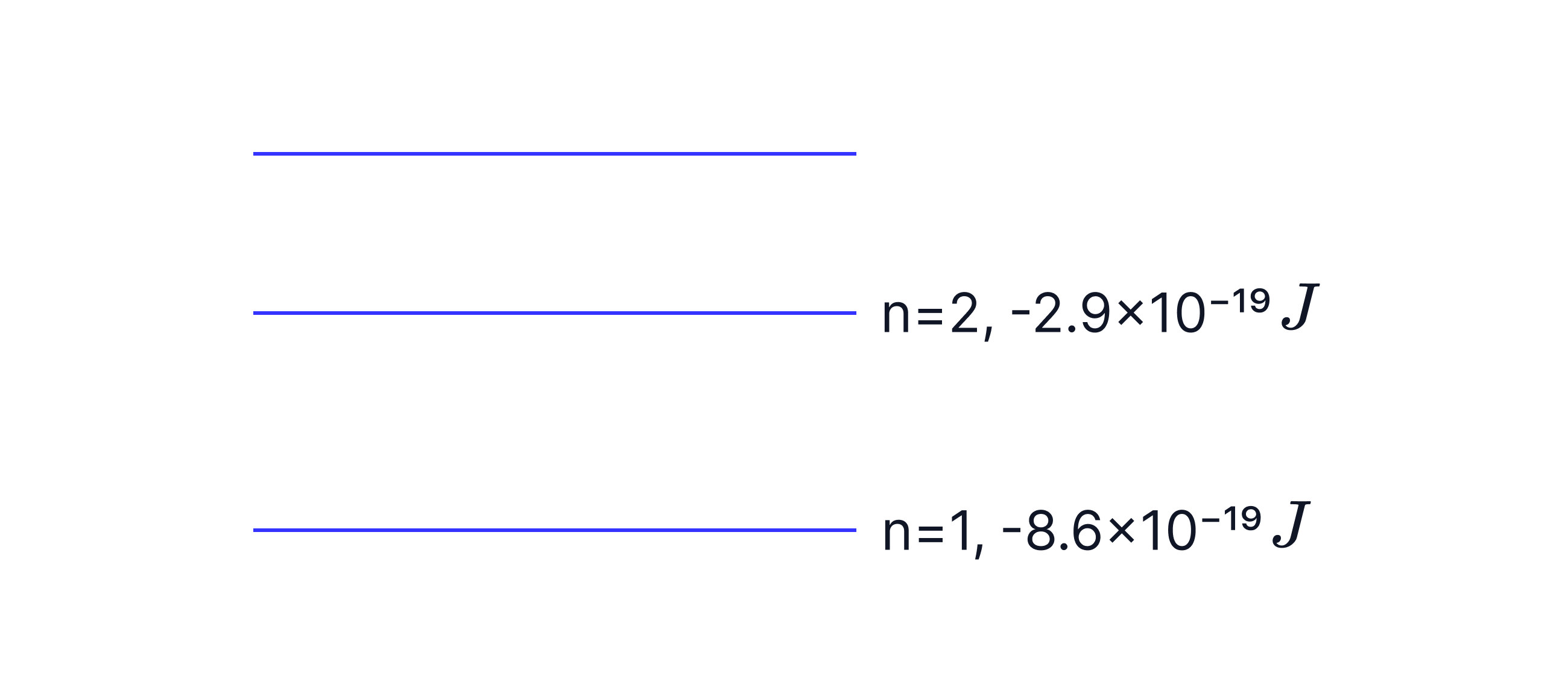
A free electron with kinetic energy collides with a stationary lithium atom in its n=1 energy level.
It is excited to the n=2 energy level.
Calculate the kinetic energy of the free electron after the collision.
-> Check out Brook's video explanation for more help.
Answer:
Mercury atoms in a fluorescent tube are excited and then emit photons in the UV region.
Explain how mercury atoms become excited.
Explain how excited mercury atoms emit UV photos.
Explain how visible photons are emitted.
-> Check out Brook's video explanation for more help.
Answers:
Free electrons moving through the tube collide with mercury atoms. Electrons in the mercury atoms absorb energy from the free-moving electrons on collision and get excited to higher energy levels.
As excited electrons in mercury atoms fall back to lower energy levels, they emit photons equal in energy to the size of the energy level gap. In mercury, the energy level gaps correspond to photon energies in the UV region.
Electrons in the atoms of the phosphor coating absorb the UV photons emitted from the mercury atoms and are excited to higher energy levels. As they fall back to lower energy levels, in stages, they emit photons in the visible region.