Thermal Energy Transfer
Brook Edgar
Teacher

Contents
Explainer Video
Thermal Energy Transfer
Internal energy is defined as the sum of the random distribution of kinetic and potential energy of the particles.
The internal energy of a substance can be increased by heating it or by doing work on the system.
This is the 1st Law of Thermodynamics.

As we heat a substance, we can monitor the temperature of the substance against time. Below is the heating curve for a pure substance that begins in the solid state.

As a substance is heated, the energy input is used to increase the substance's kinetic energy as its temperature rises. We can use the equation to calculate the increase in kinetic energy.
Formula:
Specific heat capacity is the amount of energy required to increase the temperature of of a substance by without changing state. It is a property of a material that explains why different substances lose heat at different rates. For example, water has a high specific heat capacity; it can store a significant amount of energy per unit change in temperature, which is why it is used as a coolant in nuclear reactors.
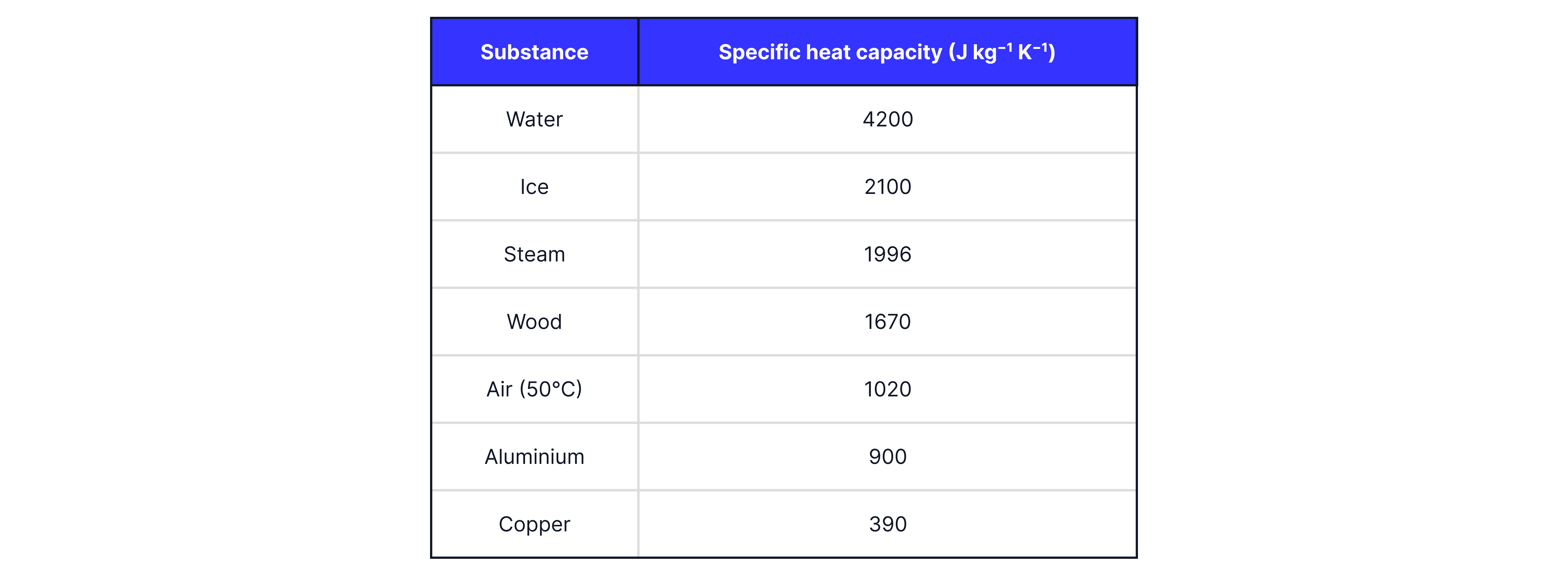
The flat line on the heating curve indicates a change in state. The energy input is used to change the potential energy of the substance. As a solid is heated, when the melting point is reached, the energy input is used to weaken or break some of the intermolecular forces between the molecules, increasing the substance's potential energy. We can use the equation to find this change in energy.
Formula:
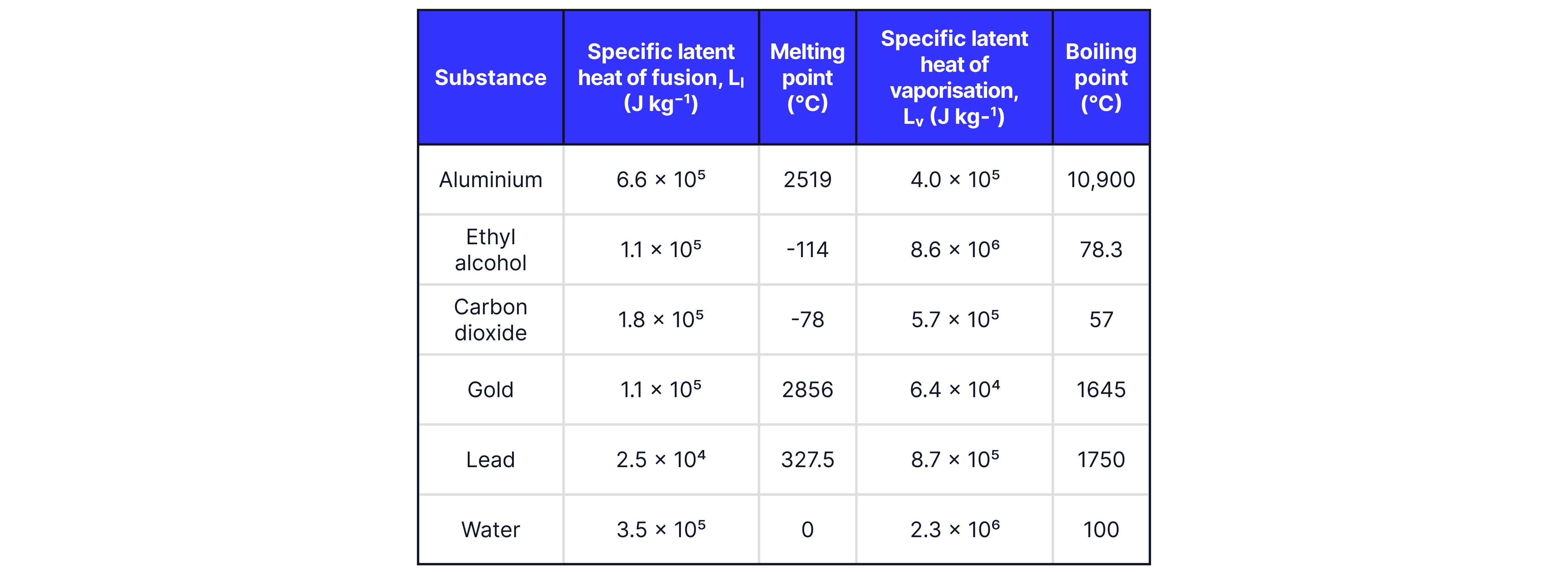
The specific latent heat of vaporisation of a substance is the energy required to change state of a unit mass of substance from liquid to gas without a change of temperature.
The specific latent heat of fusion of a substance is the energy required to change state of a unit mass of substance from solid to liquid without a change of temperature.
The SI base unit for temperature is kelvin. is known as absolute zero, which is the lowest possible temperature at which anything can exist - it is a theoretical limit that has not been reached in practice, where the kinetic energy of the particles is zero.
is equivalent to .
Remember: Water freezes at and boils at .
Energy Change Calculations
We can use specific heat capacity and specific latent heat to calculate the energy required or released for a substance to be heated or cooled, respectively.
For example, we can calculate the energy released when of water at cools to and then freezes to form ice, at .
Specific heat capacity of water =
Specific latent heat of fusion of ice =
First, we calculate the energy released as the liquid water cools from 17 to 0:
Then we can calculate the energy released as the liquid water at changes state to ice at :
To calculate the total energy drop we add the two energies:
Remember: When calculating the temperature change, we can keep the temperature in degrees Celsius, as a 17-degree change in temperature in Celsius is equivalent to a 17-degree change in temperature in Kelvin.
Worked Example
State which has higher internal energy, solid ice or liquid water?
Describe how we increase a substance's internal energy.
State the difference between and , you may use ice as an example to explain.
Define specific heat capacity.
Answer:
Liquid water, as its particles are further apart, has higher potential energy, and the particles can move around more freely, resulting in higher kinetic energy.
Heating or work done on the system. This is the 1st Law of Thermodynamics.
Zero kelvin is the coldest anything can be ( minimum internal energy) , which is equivalent to . To convert from to add .
The amount of energy required to increase the temperature of of a substance by without changing state.
Worked Example
Calculate how long it will a kettle to raise the temperature of of water from degrees Celsius to degrees Celsius.
Specific heat capacity of water = .
Answer:
Step 1. Calculate the energy required to heat the water from to degrees:
Step 2. Calculate the time needed to provide that energy:
Teacher Tips: Mass must always be in kilograms!
Worked Example
Calculate how much energy is required to melt of gold at its melting point without a temperature change.
Calculate how much energy of carbon dioxide gas needs to absorb to change into liquid carbon dioxide, at its boiling point, without a change in temperature.
Answer:
Teacher Tips: In question one, as the substance is melting, the specific latent heat of fusion is used, but in question 2, as the substance is boiling, the specific latent heat of vaporisation is used.
Practice Questions
Coke at , mass is poured into a glass at .
SHC Glass =
SHC Coke =
Calculate the final temperature of the drink.
-> Check out Brook's video explanation for more help.
Answer:
Calculate the energy released when of water at cools to then freezes to form ice at .
SHC Water =
SLH fusion of ice =
SHC Ice =
-> Check out Brook's video explanation for more help.
Answer: