Nuclear Fusion and Fission
Brook Edgar
Teacher

Explainer Video
Nuclear Fusion
Elements lighter than iron(Fe) will undergo fusion to become more stable by increasing their binding energy.
For light nuclei, increasing the number of nucleons increases the strong nuclear force, as the number of protons is relatively low, so the electrostatic force of repulsion is not dominant.
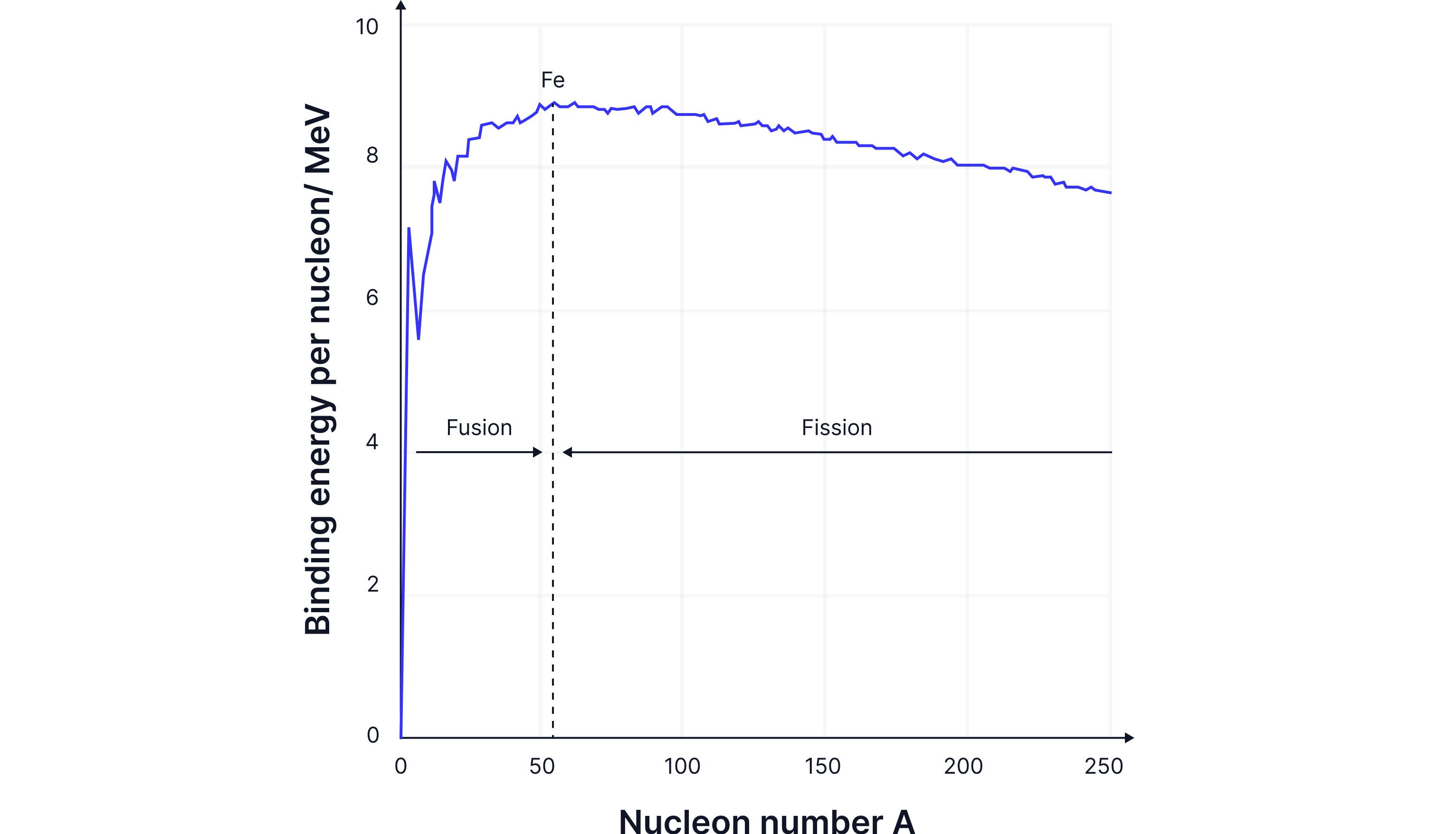
For fusion to happen, nuclei must have very high kinetic energy - they need to be moving extremely fast - to overcome the electrostatic repulsion between their positive charges and get close enough to fuse.
Fusion occurs when two light nuclei join together to form a heavier nucleus, thereby increasing their binding energy and becoming more stable. Fusion occurs in stars as the extremely high temperatures there provide the nuclei with enough kinetic energy to fuse. In the Sun, two lighter hydrogen nuclei fuse to form a heavier helium nucleus, releasing a vast amount of energy.
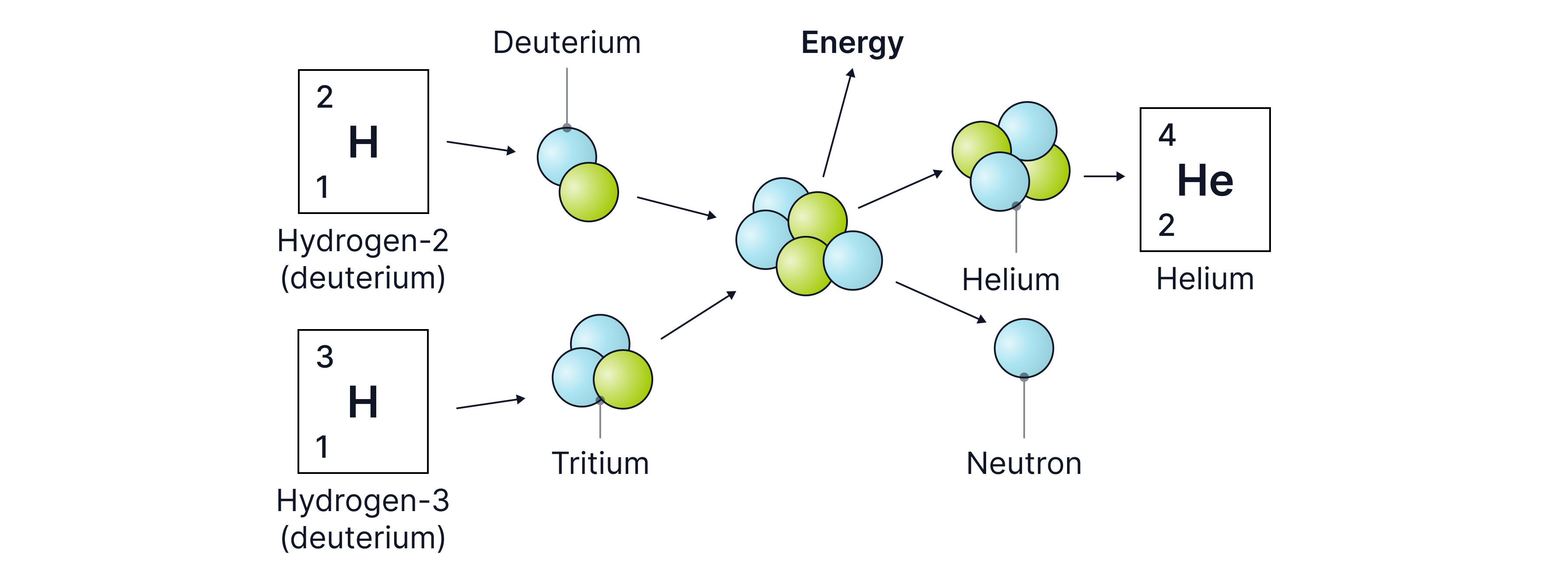
Research into fusion reactors began in the 1940s, but no design has produced positive net energy. There are several challenges in building a net energy-positive fusion reactor:
A significant amount of energy is required to heat the material to a superhot plasma state so the kinetic energies of the nuclei are high. This requires very high electric currents.
The plasma must be contained by a powerful magnetic field so that it does not come into contact with the reactor walls (if it did, the plasma would cool, transferring heat to the container and thus losing kinetic energy, which would stop the reaction).
Nuclear Fission
Elements heavier than iron nuclei are less stable, as the large number of protons increases the electrostatic repulsion force within the nucleus, and the strong nuclear force has less of an effect due to the larger nucleus. Therefore, the binding energy per nucleon decreases, as less energy is needed to remove a nucleon from the larger nucleus.
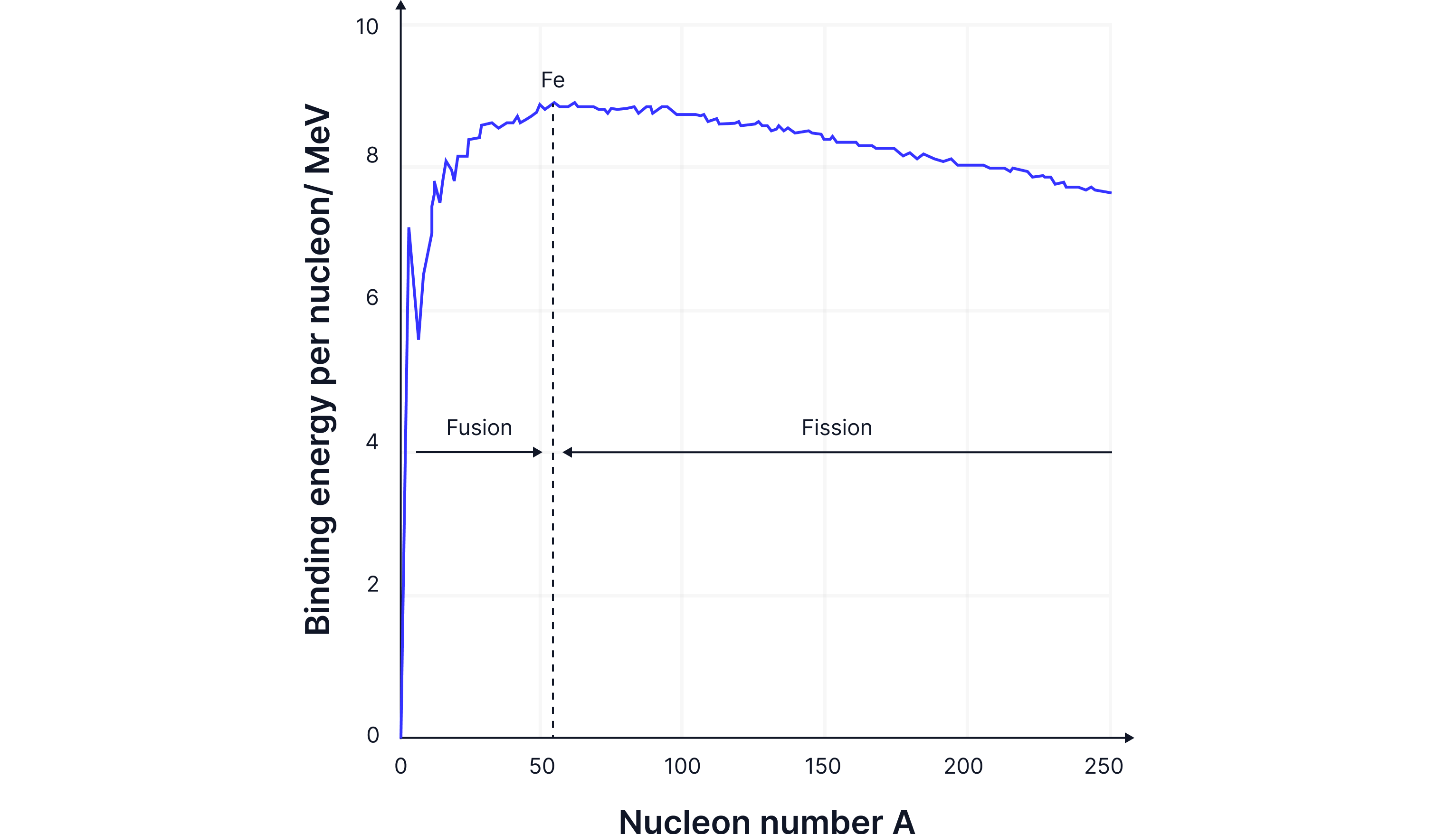
Large nuclei, therefore, undergo fission to become more stable, to increase their binding energy per nucleon. The large nucleus breaks into two smaller nuclei that have a higher binding energy.
We can induce fission by firing a neutron at a Uranium-235 nuclei. The neutron is absorbed, causing the nucleus to split into two lighter nuclei, releasing energy and more neutrons.

The emitted neutrons can go on to cause further fission events in other Uranium nuclei, which can lead to an uncontrolled reaction and potentially an explosion. Nuclear reactors operate at or slightly above critical mass - the smallest amount of fissile material needed to sustain a self-sustaining nuclear chain reaction, where the number of neutrons produced = the number of neutrons lost.
Spontaneous fission occurs when the nucleus splits without a neutron being absorbed. This is rare.
Energy released in Fission
To calculate the energy released in fission, we need to calculate the difference in binding energies.
Uranium-236 undergoes nuclear fission to produce barium-144, krypton-89 and three free neutrons.
The binding energies per nucleon are given here, so we need to multiply by the total nucleon number/mass number to calculate the total binding energy per nucleus first in order to find the differences.
Nuclide | Binding energy per nucleon () |
|---|---|
We do not need to include the three neutrons here, as neutrons have zero binding energy.
To find the change in binding energies, we subtract the total binding energy of the parent nuclei from the total binding energy of the products, as the two daughter nuclei have a higher binding energy per nucleon, as fission always occurs to increase the binding energy per nucleon.
This change in energy is the energy released. The energy comes from the change in mass as the total mass of the products is always less than that of the reactants. This difference in mass is converted to energy, . The products have a higher binding energy as they are more stable; more energy would be required to break these nuclei up into their individual nucleons.
Worked Example
Calculate the maximum energy in released when of Li- is hit with neutrons.
Nuclei | Mass (u) |
|---|---|
Neutron | |
Hydrogen- | |
Helium | |
Lithium |
Answer:
Teacher Tips: In fusion, energy is released as there is a change in mass. The mass of the products is less than the mass of the reactants, and this missing mass is released as energy. Take care that if they give you the mass in atomic mass units, you need to convert this into kg.
Remember: You only ignore neutrons in the equations when working with binding energies, as they have zero binding energy (no energy is required to break lone neutron, as it is already on its own). If calculating changes in mass, you need to include them, as neutrons have mass. Also, beware that in exam questions, they can give you the binding energy of the nucleus or the binding energy per nucleon. If the latter is given, you need to multiply by the total nucleon number/mass number.
Practice Questions
Explain why nuclei that undergo fission are on a different part of the Binding Energy graph than those that undergo fusion.
-> Check out Brook's video explanation for more help.
Answer:
Nuclei lighter than Iron-56 undergo fusion. Light nuclei join together to make a heavier nucleus to increase their binding energy per nucleon.
Nuclei heavier than Iron-56 undergo fission. A heavier nucleus breaks into two lighter nuclei, increasing the binding energy per nucleon.
One model of nuclear fusion suggests that fusion happens when nuclei touch. Fusion occurs when the atoms are separated by .
Calculate the total change in electrostatic potential energy between the initial and final positions.
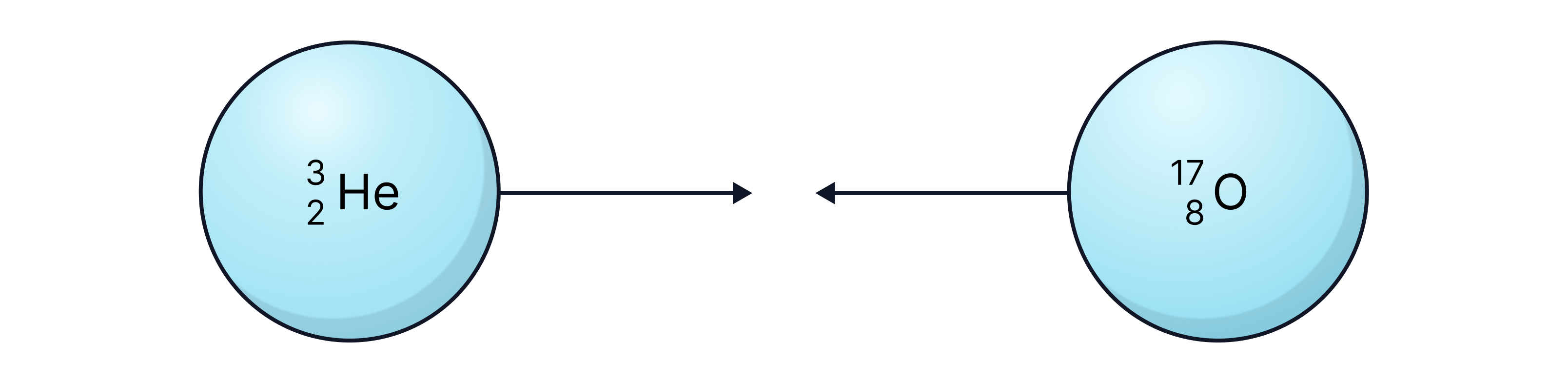
-> Check out Brook's video explanation for more help.
Answer:
Energy released from fission of a U-235 nuclei is .
Compare the energy released from the fission of U-235 to the energy released from the fusion of of Hydrogen-2.
The fusion of two Hydrogen-2 nuclei releases .
Hydrogen-2 mass = u
-> Check out Brook's video explanation for more help.
Answer:
Fusion releases more energy.
Uranium =
hydrogen =