Nuclear Radius
Brook Edgar
Teacher

Contents
Explainer Video
Back To Rutherford
We can estimate nuclear radius by calculating the distance of closest approach of a charged particle.
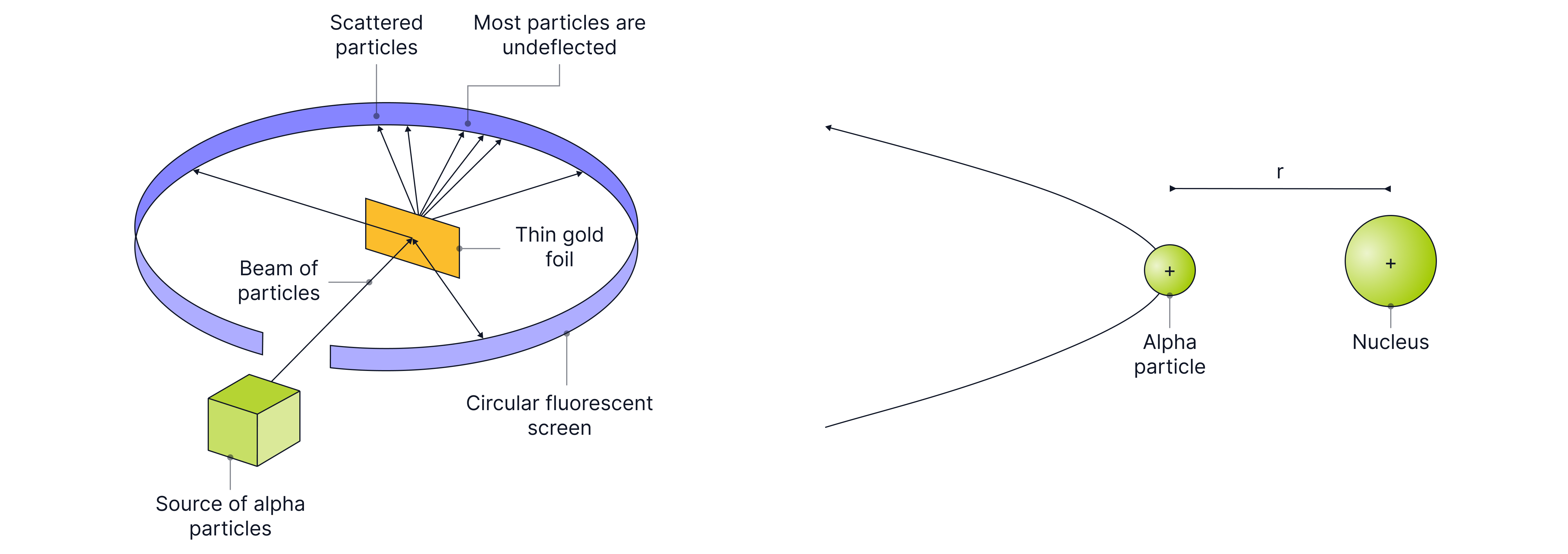
Rutherford fired alpha particles at a thin gold foil (a few atoms thick). He saw that as the alpha particles approached the gold nucleus, they experienced a force of electrostatic repulsion, causing them to lose kinetic energy and eventually change direction due to the increase in potential energy from the increasing repulsion.
At the closest approach, all of the alpha particles' initial kinetic energy is converted into electric potential energy due to energy conservation.
To calculate the electric potential energy at the distance of the closest approach, we use an equation from the electricity and electric fields chapters.
Formula:
Formula:
Combining the two equations:
Teacher Tips: The two Qs are different - one is the charge being moved towards the large nucleus ; the other is the nucleus's own charge.
Worked Example
An alpha particle is fired at a gold nucleus ( protons) with of energy. The distance of closest approach of the alpha particle can give us an approximation for the size of the nucleus.
The alpha particle is brought to rest at point S, a distance r from the centre of the nucleus. Calculate the distance of closest approach.
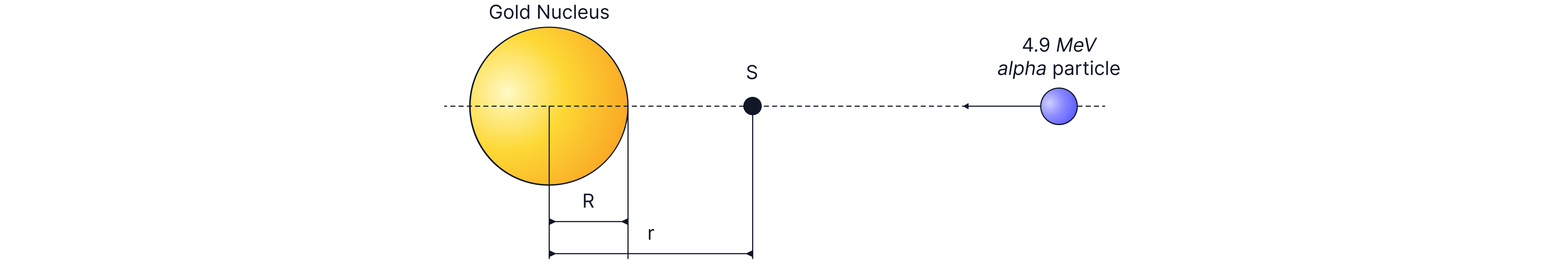
Answer:
Step . Convert eV to joules to find the initial kinetic energy:
Step Equate the kinetic energy to the electric potential energy, and solve for r:
Teacher Tips: Remember that the relative charge of an alpha particle is as it has two protons, and the relative charge of the gold nucleus is as it has protons.
High Energy Electron Diffraction
Rutherford's method always gives an overestimate of the nuclear radius, as it gives the distance of closest approach.
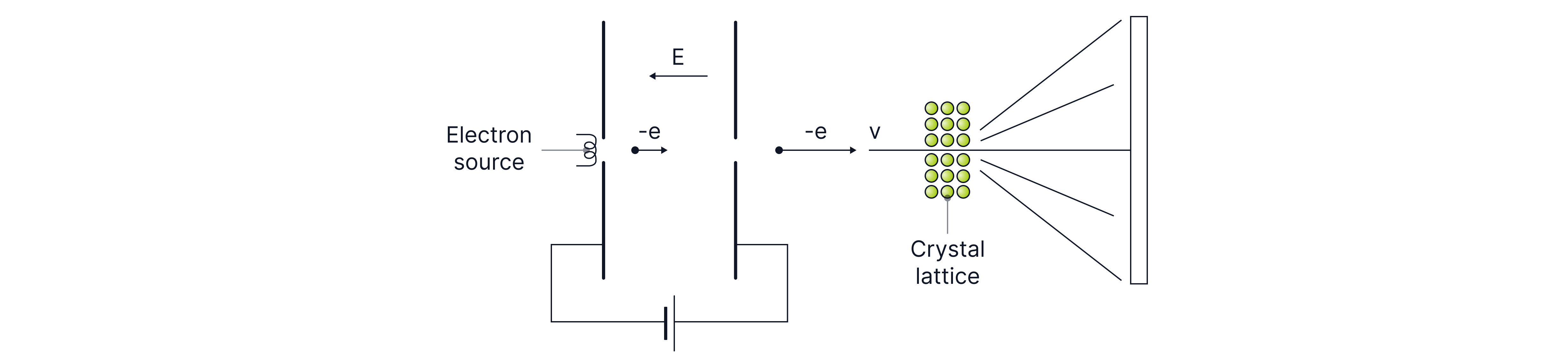
Instead, to find the nuclear radius, we can use high energy electron diffraction, where electrons are fired at a crystal lattice, diffracted around the nuclei of atoms to form an interference pattern on the screen. This is a more accurate method for determining nuclear radius, as electrons are leptons (paper 1), so we know that they will not interact with the nucleons in the nucleus through the strong nuclear force, unlike the alpha particle. Electrons can also get much closer to the nucleus as they have less recoil due to their small mass, and they do not experience the electrostatic force of repulsion as they have a negative charge.
Remember: The pattern on the screen is a set of concentric circles with a bright central spot, as when electrons diffract around the nucleus, they act as waves and superpose. Constructive interference occurs at the central maximum, as the path difference is zero and as the waves are exactly in phase, the phase difference is .
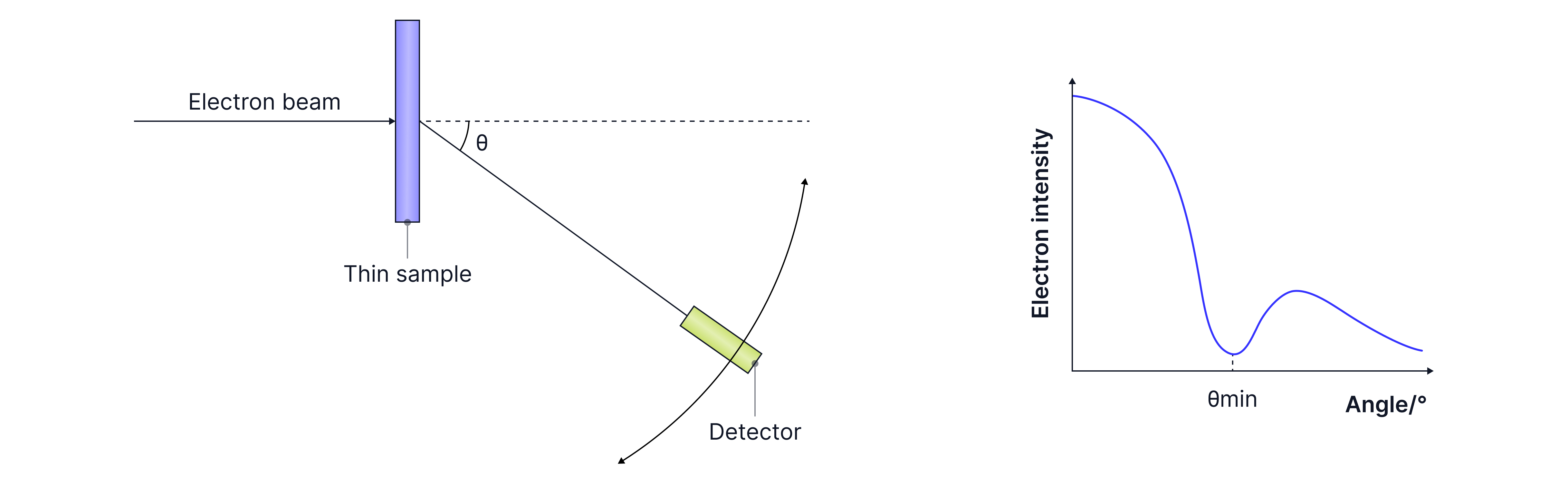
In high energy electron diffraction, the electrons are accelerated to high speeds across a potential difference and diffract by the nuclei of atoms as their De Broglie wavelength ~, is on the same order of magnitude. The angle to the first minimum on the diffraction pattern formed on the screen gives us the nuclear radius.
Formula:
The equation to find nuclear radius is similar to the equation from the diffraction grating experiment (paper 1), but as the nucleus is not a regular, periodic structure like the evenly spaced slits in diffraction gratings so the equation does not apply exactly.
Assuming that the nucleus is spherical and all nucleons are packed with a constant density the volume of a nucleus should increase with atomic number, A.
The experiment was repeated for different nuclei, and from a logarithmic graph, we can determine the relationship , proving that nuclear density is constant.
is a constant ~ .
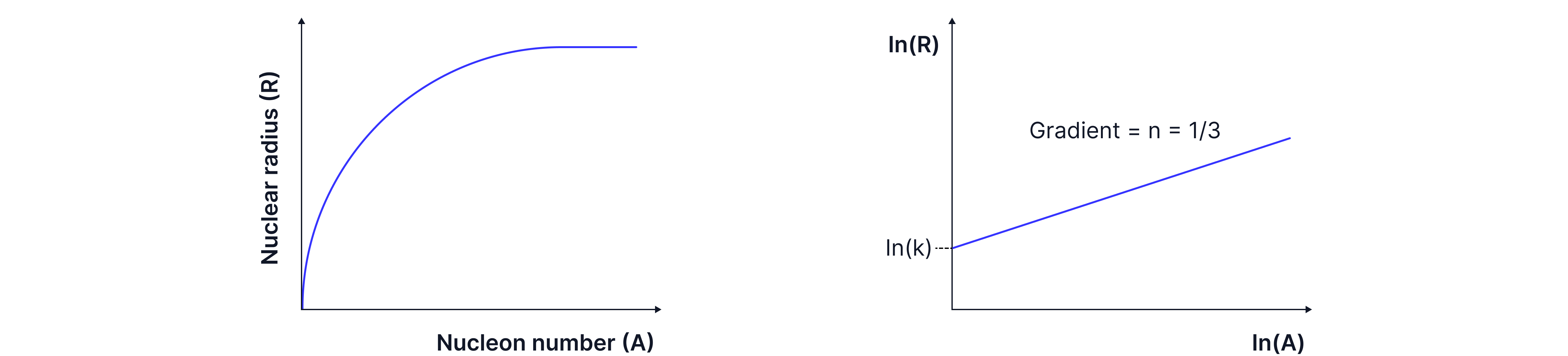
Using the nuclear radius equation, , we can show that nuclear density is constant:
The terms in the final density equation are all constants. Showing that the density of a nucleus is independent of the nucleon number.
Worked Example
Explain why electron diffraction is a better method than alpha scattering to determine nuclear radius.
Answer:
Previously, we estimated the nuclear radius by using the distance of closest approach when an alpha particle was fired at a nucleus, equating the kinetic energy of the alpha particle to the electrical potential energy. However, this value always gives an overestimate, too large.
Electrons are leptons; they will not interact with the strong nuclear force in the nucleus like the alpha particle would. Electrons also have less recoil as their mass is small and can get much closer to the nucleus, as they are negatively charged, so the positively charged nucleus won’t repel them, improving the accuracy of the nuclear radius measurements.
Worked Example
Sketch a labelled diagram showing the equipment used in the alpha scattering experiment that Rutherford used to provide evidence for the structure of the atom.
Answer:
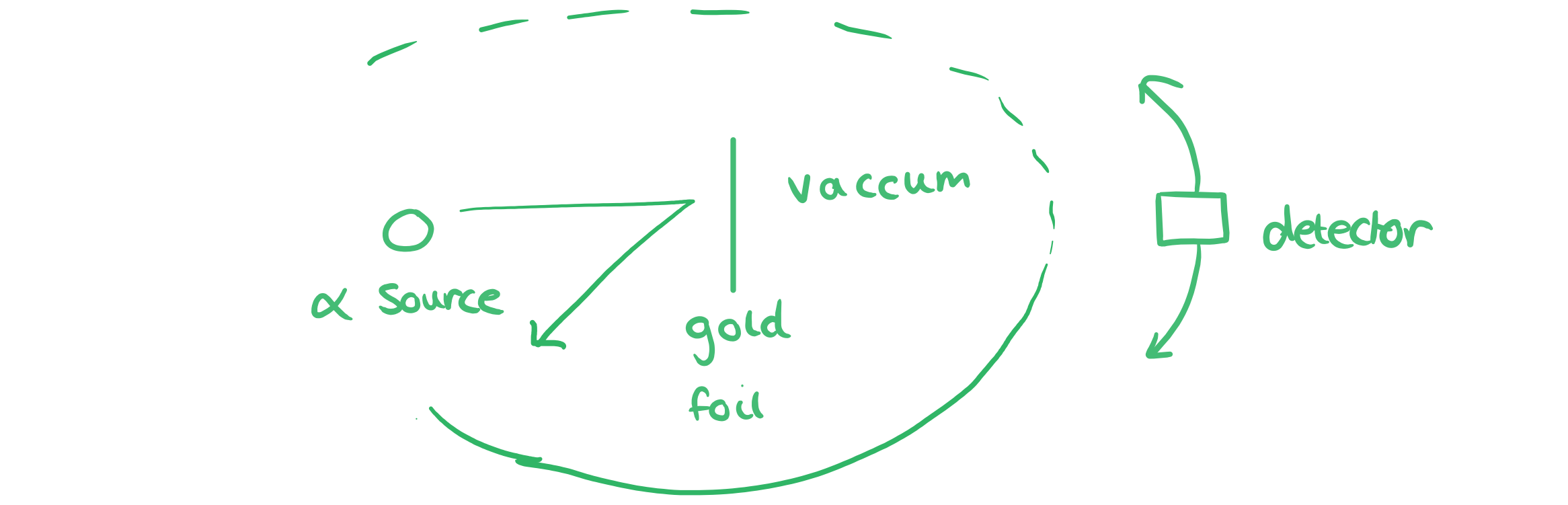
You must make sure you include the label ‘vacuum’, state that there is gold foil, and that the detector can move all the way around in a circular path, as alpha particles can be backscattered.
Practice Questions
Calculate the distance of closest approach. The gold atom, Au, has protons.
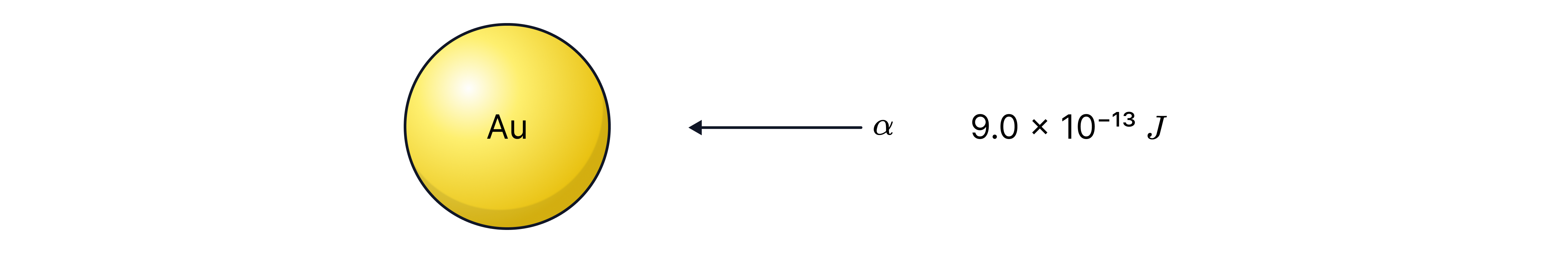
-> Check out Brook's video explanation for more help.
Answer:
has a nuclear radius of . Determine the radius of .
-> Check out Brook's video explanation for more help.
Answer: