Osmosis
Laura Armstrong & Joe Wolfensohn
Teachers


Contents
Recall Questions
This topic requires prior knowledge of diffusion to explain the movement of water across membranes.
What is diffusion?
The net movement of particles from higher to lower concentration.
What is the function of the cell membrane?
To control the movement of substance into and out of the cell.
What does “concentration gradient” mean?
The difference in concentration between two areas.
Topic Explainer Video
Check out this @lauradoesbiology video that explains osmosis, then read the study notes. Once you’ve gone through them, don’t forget to try the practice questions!
Osmosis
What Is Osmosis?
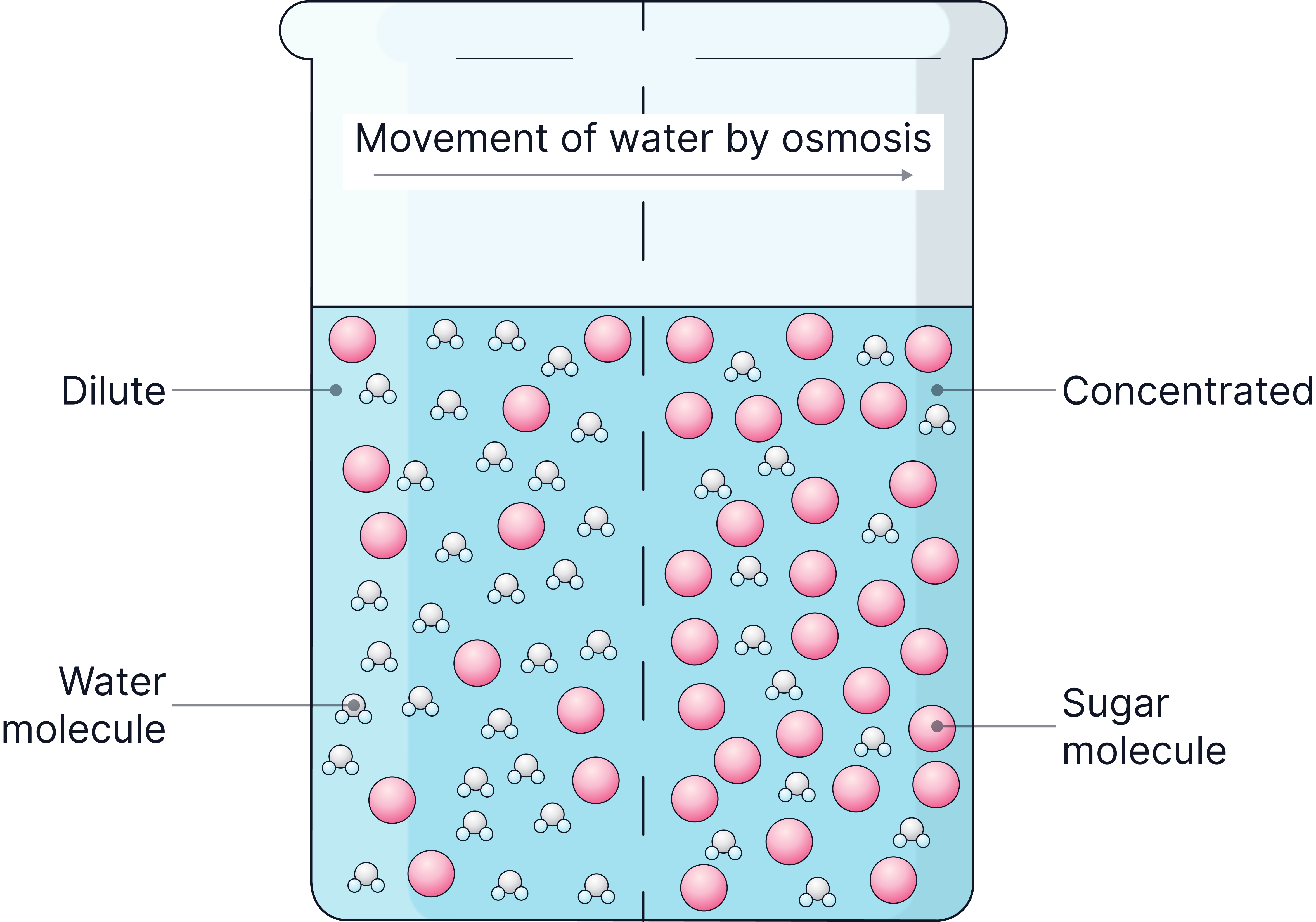
- Osmosis is the diffusion of water across a partially permeable membrane, from a dilute solution to a concentrated solution.
A dilute solution will have a higher concentration of water, with a lower concentration of solute dissolved in it.
A concentrated solution will have a higher concentration of solute, for example, more salt or more sugar dissolved in the water. - Osmosis is a passive process – no energy from respiration is needed.
- The cell membrane is an example of a partially permeable membrane. Water can pass through it easily and it controls the movement of sugar or salt particles.
Where Osmosis Happens
- Root hair cells – water moves from soil into root cells.
- Animal cells – gain or lose water depending on the surrounding solution.
- Plant cells – water moves into or out of the vacuole affecting pressure inside the cell.
Effects of Osmosis in Cells
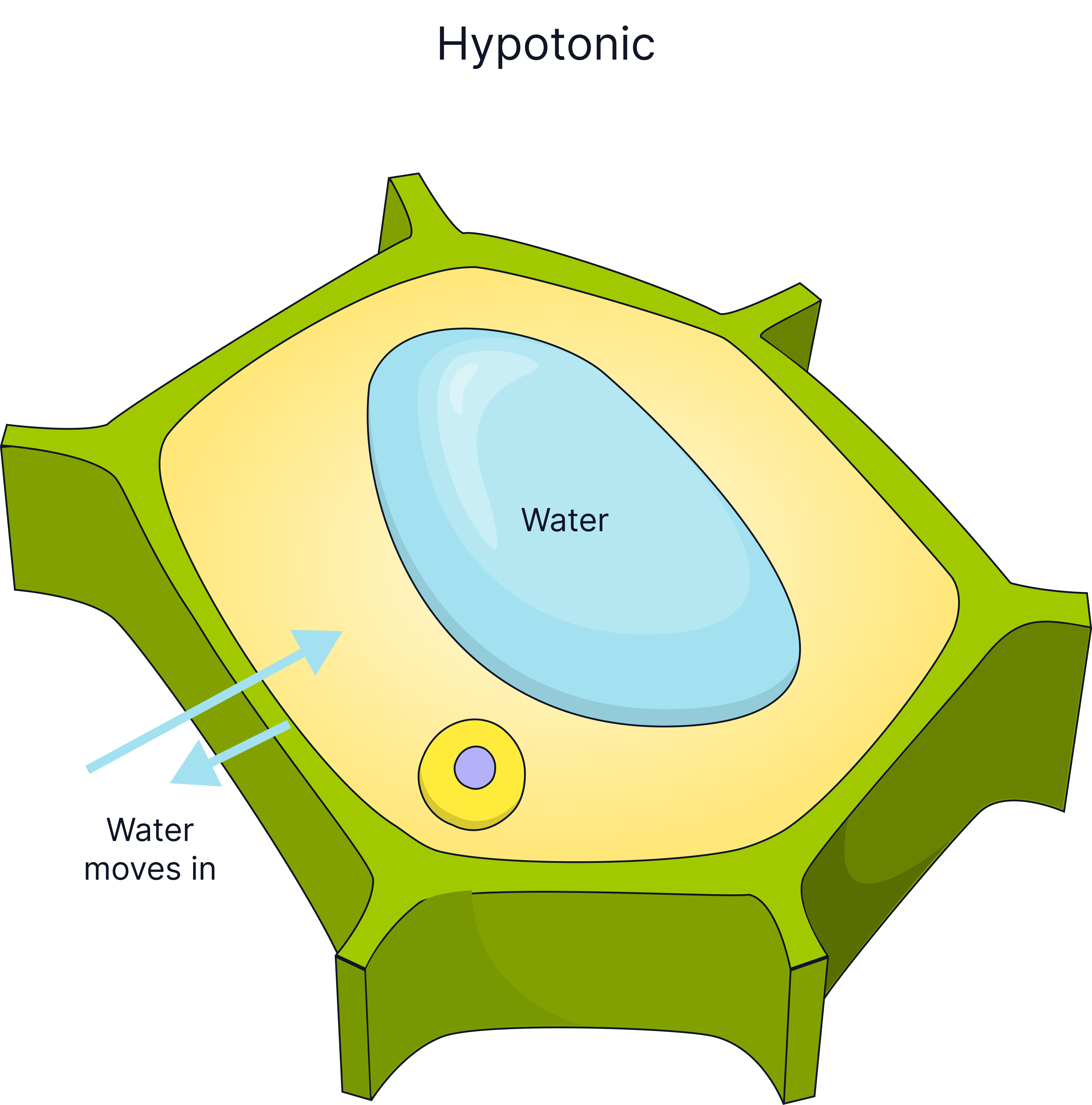
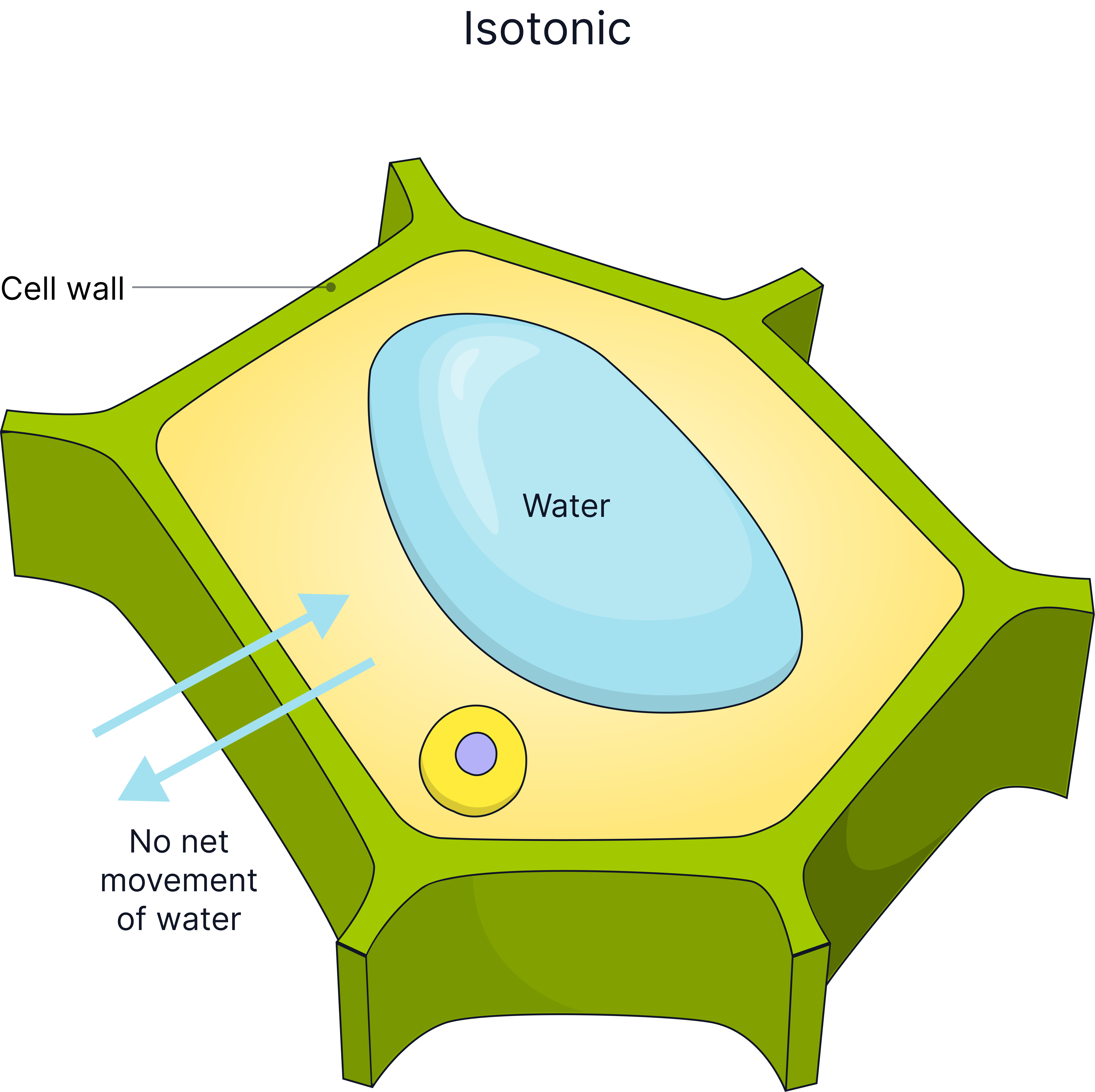
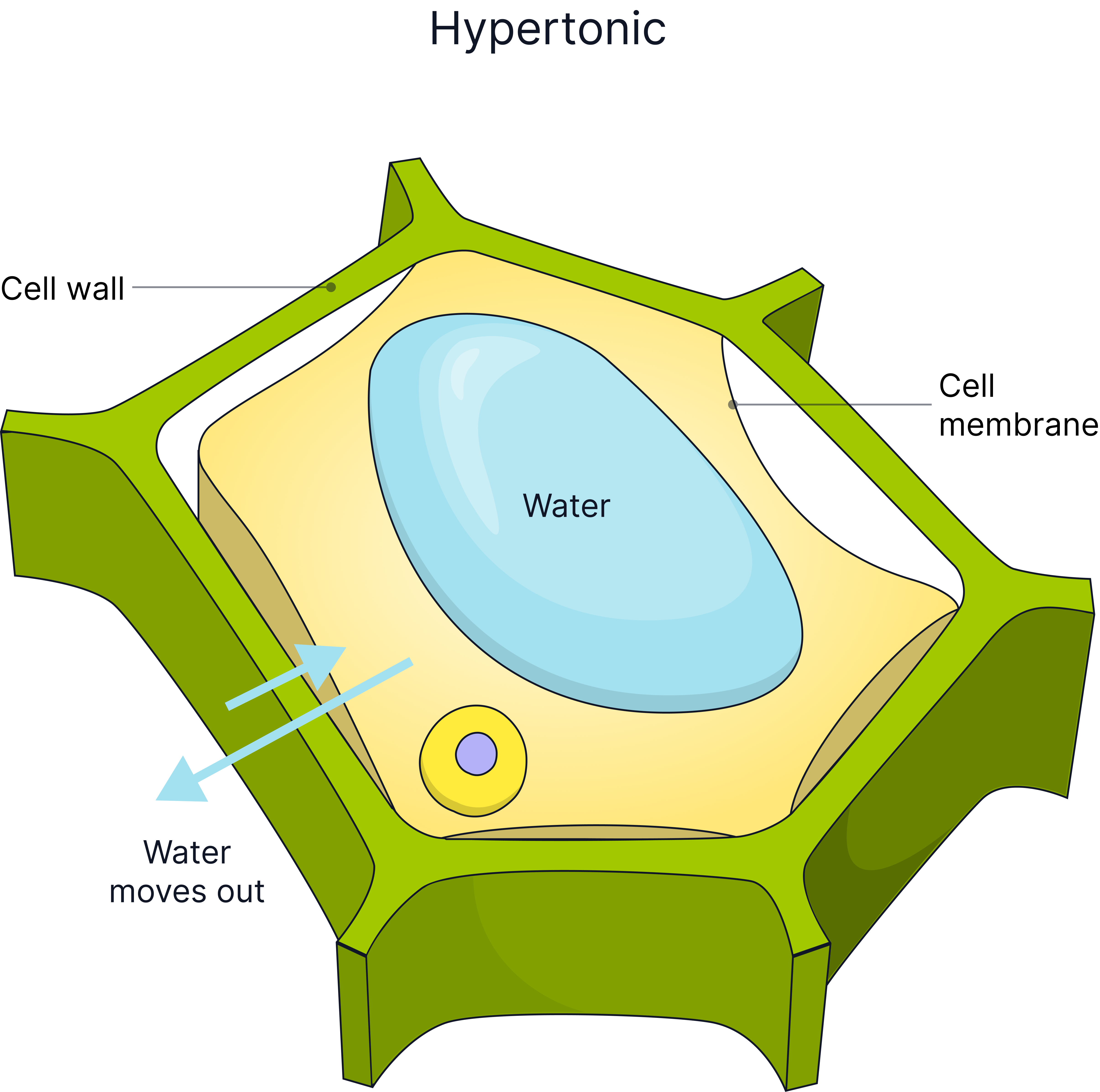
Cells in different concentration solutions will lose or gain water and so will gain or lose mass.
|
Cell Type |
In Pure Water (Hypotonic) |
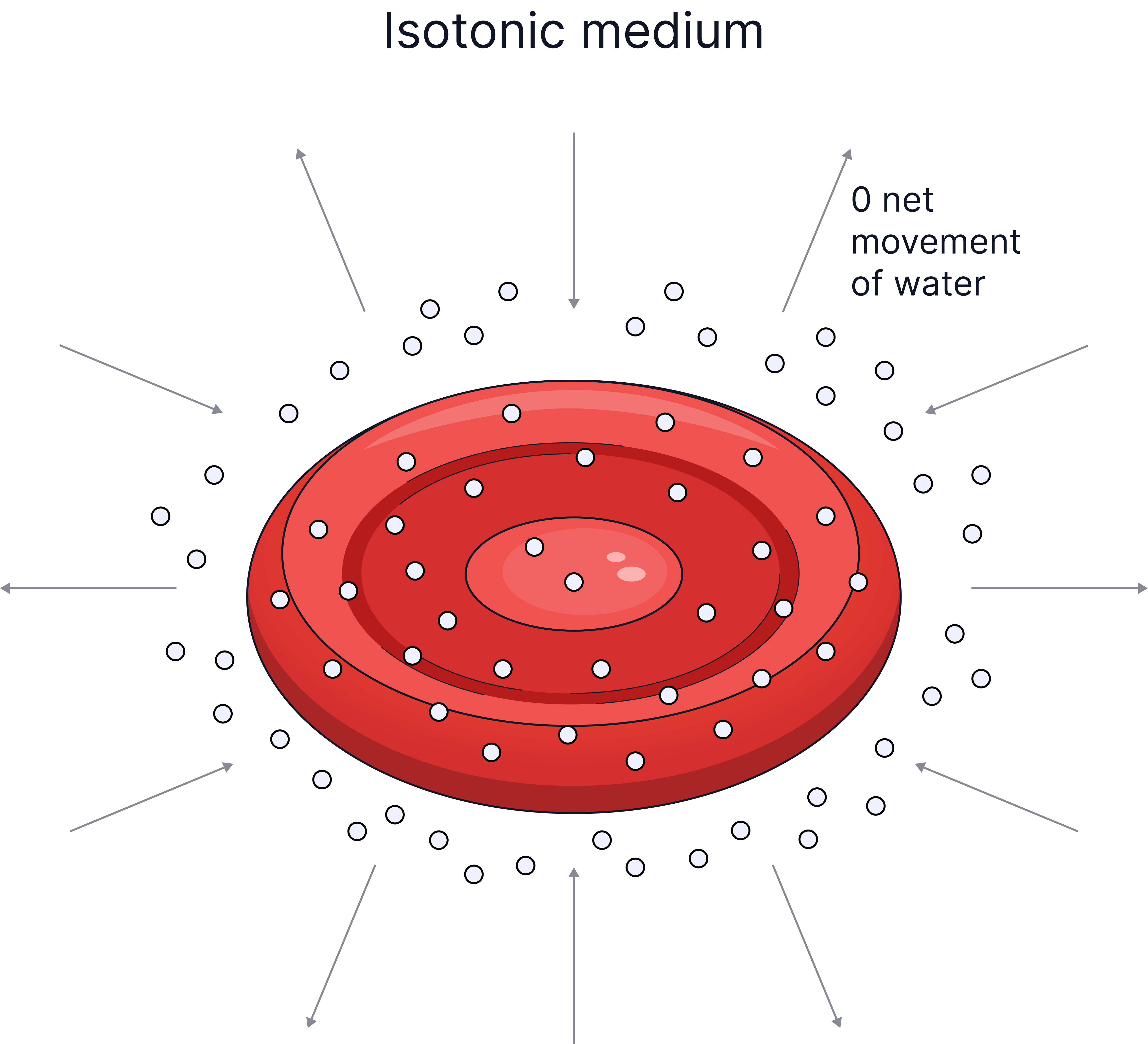
In a solution the same concentration as inside the cell (Isotonic) |
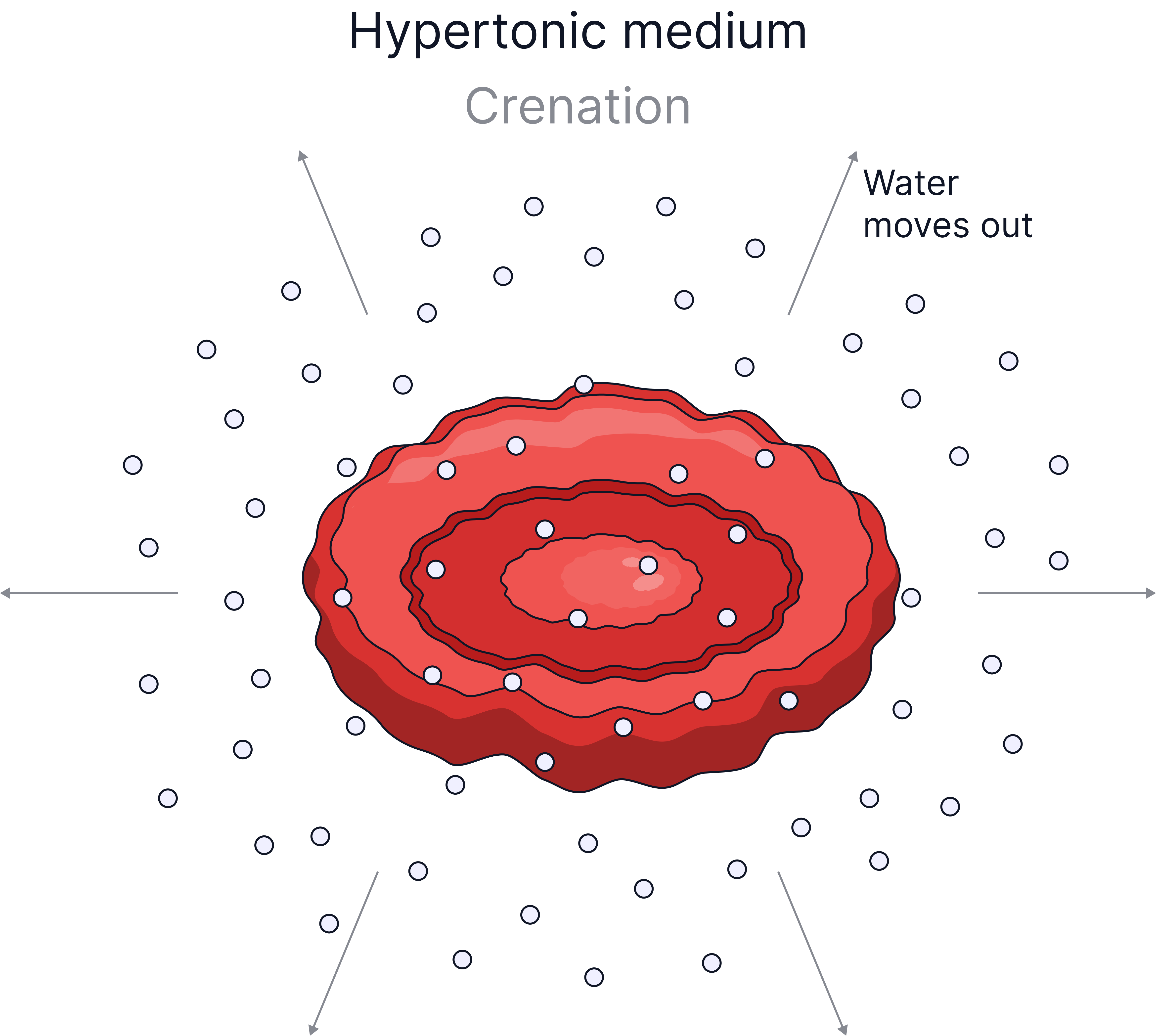
In Concentrated Solution (Hypertonic) |
|
Plant |
Water enters cell, cell gains mass, cell becomes turgid. |
0 net movement of water, no change in mass of cell. |
Water leaves cell, cell loses mass, cell becomes flaccid or plasmolysed (cell membrane pulls away from the cell wall. |
|
|
|
|
|
|
Animal |
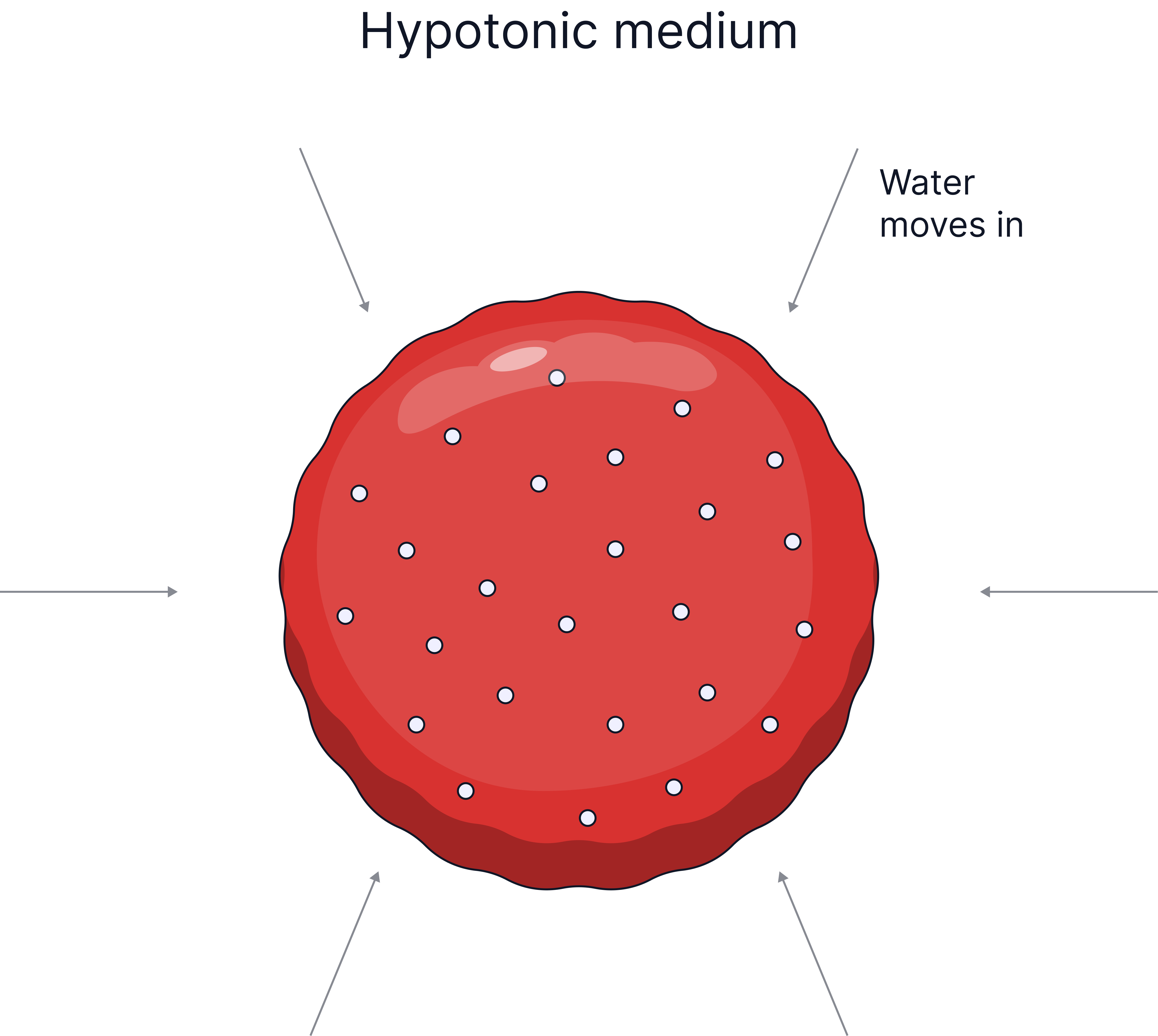
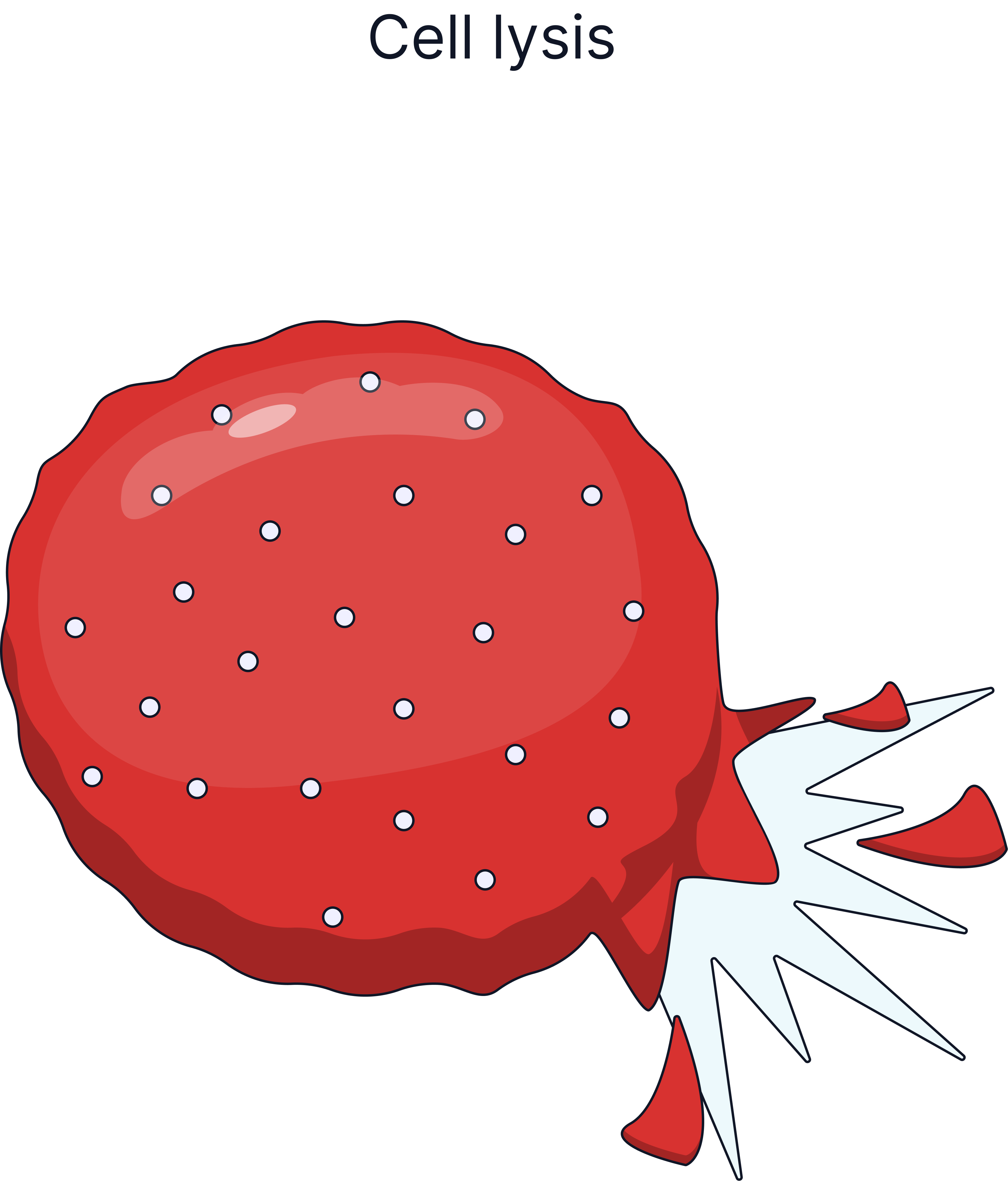
Water enters, cell gains mass, cell may burst (lysis), because there is no cell wall. |
0 net movement of water, no change in mass of cell. |
Water leaves, cell loses mass, cell shrinks / shrivels (crenates). |
|
|
|
|
|
Plant cells have a cellulose cell wall which can withstand the pressure exerted when water enters the cell, this is why plant cells do not burst when placed in pure water, they become turgid. Animal cells lack a cell wall, so if too much water enters by osmosis, for example, into a red blood cell, the cell will burst.
The plant cell wall is fully permeable to water.
Factors Affecting the Rate of Osmosis
Just like diffusion, osmosis is influenced by several key factors that affect how quickly water moves across a partially permeable membrane:
1. Concentration Gradient
- A steeper gradient (greater difference in water concentration) = faster osmosis.
2. Temperature
- Higher temperatures give water molecules more kinetic energy, increasing their movement = faster osmosis.
3. Surface Area of the Membrane
- Larger surface area = more space for water to pass through = faster osmosis.
4. Thickness of the Exchange Surface
- Thinner exchange surface = shorter diffusion path, so water crosses more quickly = faster osmosis.
Key Terms
- Osmosis – movement of water through a partially permeable membrane, from a dilute to a more concentrated solution.
- Partially permeable membrane - a membrane that only some particles can pass through.
- Isotonic solution - a solution with the same concentration of water.
- Hypotonic solution - a solution with a lower concentration of solute, it is more dilute.
- Hypertonic solution - a solution with a higher concentration of solute, it is more concentrated.
Exam Tip
- Always state that water moves by osmosis to ensure you get the marks. Remember, water will move from a more dilute to a more concentrated solution.
Practice Question
Describe what would happen to a red blood cell placed in pure water and explain why. (3 marks)
Model Answer:
Water will move into the red blood cell by osmosis.
This happens because the area outside the cell is more dilute than inside the cell.
The cell will gain mass.
The cell may swell and burst (lysis) because it has no cell wall.
More Practice
Try to answer these practice questions from the TikTok videos on your own, then watch the videos to see how well you did!